Hover over buttons to get to the sub-pages
This Month's Featured Story
Handy Reference Poster to the 50 State Gemstones

Here is a great poster for you to open up for reference or download to keep for yourself. It's from the great website - Vivid Maps (https://vividmaps.com/us-minerals-mapped/). A very useful and interesting place to go down a rabbit hole. If you get a chance, go check them out. All kinds of interesting maps, infographics, and content. Credit to Esri Aubri, a software engineer, who made this vivid map.
Previously Featured
As our technologies keep improving, it leads to more startling and amazing discoveries as to what CAN BE fossilized and what we might be able to detect and deduce in future studies of fossils. It seems that we can now detect the remains of fossilized blood, metabolic rates, chemical signatures of some pigments in skin and feathers, and, in some rare cases, even internal soft tissue organs have been studied in 66-million-year-old fossils.
New T.Rex Species Confirmed and Much More!
This twenty-two-minute presentation covers a wide and amazing array of subjects regarding Tyrannosaur fossils and new science on what can be fossilized that was once thought impossible.
Do Minerals Evolve?
Dr. Hazen was asked a simple question. "Was this mineral around back a long time ago?" This led to a paradigm shift in how he looked at minerals and what evolution is. Does evolution apply only to life, or can it be applied as a natural law across all natural systems? Applying the ideas of evolution to the mineral world has brought him to a new way of seeing minerals and the laws of nature.
This is about a 50-minute presentation from Dr. Robert Hazen's observations and scientific research into many natural processes.
Minerals Used to Build the Case for Life on Early Mars
Minerals found in the Jezero Crater by the Perseverance rover, specifically vivianite (iron phosphate) and greigite (iron sulfide), are considered potential biosignatures for ancient microbial life. These minerals form through redox reactions that occur when organic matter is consumed by microbes, producing the minerals as byproducts.The specific arrangement and chemical signatures of these minerals, alongside evidence of oxidized iron being consumed, strongly suggest past biological activity.

Leopard spotted Rock found in Jerico Crator. Photo credit to NASA.
Why Vivianite and Greigite are Key:
-
Redox Reactions: On Earth, the formation of vivianite and greigite is often linked to redox (electron-transfer) reactions carried out by microbes. These reactions are fundamental to how living organisms, including microbes, get energy from their environment.
-
Organic Matter: The presence of organic carbon within the mineral spots, combined with the minerals, supports the hypothesis that ancient microbes utilized the organic matter in the mud as an energy source, leading to the precipitation of vivianite and greigite.
-
Leopard Spot Pattern: The minerals appear in distinct "leopard spot" patterns within the rock. These spots show signs of being bleached of their original red color because the redox reactions consumed the oxidized iron, leaving behind the iron-rich, lighter-colored minerals.
Context is Crucial
-
Lakebed Habitat: The rover found these minerals in mudstones within the Jezero Crater, an ancient lakebed environment known to be conducive to microbial life.
-
Abiotic Explanations: While these minerals can be produced by nonbiological processes (e.g., high temperatures or acidic conditions), the specific combination with organic matter and the bleaching pattern makes a biological explanation the most compelling.
Sources: https://www.nasa.gov/news-release/nasa-says-mars-rover-discovered-potential-biosignature-last-year/#:~:text=In%20higher%2Dresolution%20images%2C%20the,and%20binding%20by%20organic%20compounds, https://www.space.com/astronomy/mars/the-metal-detector-has-gone-off-perseverance-rovers-find-is-a-shiny-new-clue-in-the-search-for-life-on-mars, https://en.wikipedia.org/wiki/Vivianite, https://www.mindat.org/min-1747.html,
Forget About Diamond, We Want SUPER Diamonds !
We are always skeptical when scientists start talking about improving upon what nature has created, and finding substances harder than diamonds has been the holy grail for decades. But what if you could improve upon diamonds but making them grow more consistently, without flaws, and without impurities? Well, you might have something that is a diamond, but actually harder and better.
There has been quite a lot of talk about lab-created diamonds and the value of man-made vs. natural diamonds, but perhaps our focus on pretty rocks is not the real story? This 45-minute video does a great job of presenting how lab-created diamonds were invented, improved upon, and how they could change the world.
For our January story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. These two are very similar in name and often confuse collectors because of their names and appearance.
So what's it gonna be?
Schorl vs Schorlomite
While both minerals can appear very similar, they have different mineral habits. They are visually very similar and do share some common chemistry, but we have not seen them form together.
Schorl
Formula: NaFe³⁺₃Al₆(Si₆O₁₈)(BO₃)₃(OH)₃(OH) - Tourmaline Group
-
Schorl is a mineral species within the Tourmaline Group.
-
It is regarded as an iron-rich variety of Tourmaline.
-
Comes in around 7.0 to 7.5 on the Mohs hardness scale.
-
It is the most common form of Tourmaline and of often called black tourmaline.
-
It has an opaque, black columnar crystal structure and is sometimes cut into gemstones.
Scholomite
Formula: Ca₃(Ti,Fe³⁺,Al)₂[(Si,Fe³⁺,Fe²⁺)O₄]₃ -
Garnet Supergroup
-
Schorlomite is a mineral species within the Garnet group.
-
It is regarded as a titanium-rich variety of Andradite.
-
Comes in around 7.0 to 7.5 on the Mohs hardness scale.
-
Also known as black garnet, but can be easily confused with Melanite.
-
It has an opaque, shiny black geometric crystal structure and is sometimes cut into gemstones.
Schorl (black tourmaline) and schorlomite (a titanium-rich garnet) can appear very similar due to their black color and vitreous to sub-metallic luster. However, several key differences can help differentiate them:
1. Crystal Habit
-
Schorl typically forms prismatic or needle-like crystals, often exhibiting striations along their length. It can also occur in radial, columnar, or massive forms. Cross-sections of schorl crystals will appear triangular.
-
Schorlomite is usually found as rounded grains or massive granular aggregates, but can also form dodecahedrons or trapezohedrons. It crystallizes in the isometric (cubic) system, though it can rarely form actual cubes.
2. Streak
-
Schorl produces a brownish streak.
-
Schorlomite typically yields a colorless streak, though some sources mention a black to red-orange or orange-brown streak.
3. Cleavage and Fracture
-
Schorl: Has indistinct cleavage and conchoidal fracture.
-
Schorlomite: Lacks distinct cleavage and exhibits conchoidal fracture.
4. Optical Properties
-
Schorl is uniaxial negative and exhibits strong pleochroism (appears to change color when viewed from different angles).
-
Schorlomite is isotropic (does not exhibit pleochroism) with a high refractive index (n = 1.94 - 1.98).
5. Density
-
Schorl has a density of 3.1 - 3.2 g/cm³.
-
Schorlomite is a bit denser, with a density of 3.81 - 3.88 g/cm³.
Even using the comparisons, both minerals have a similar appearance, identical hardness, and can appear very similar depending on the specimen. While Schorlomite will seem a bit heavier in the hand, it would be very difficult to know without some way to test the density. The lack of clevage in garnets and Troumaline leaving a white steak might be the easiest way to test between the two. Happy hunting out there!
Sources: Mindat.com, Geology.com, and Google AI
Biological Crystals, Microtubules, Anesthesiology and Quantum Consciousness?
OK... OK. This one is a bit on the metaphysical side, on the fringe, and you have to just listen to it and eventually, it comes together pretty well. The story eventually makes "sense" after some meandering around. It's 23 minutes, and you might think it's crazy towards the middle, but stick with it because it's worth it.
It outlines how tryptophan biologic crystals form complex structures called microtubules. These micro-tubes could form a basis behind quantum effects, and how Arthur Penrose postulated that this cellular engineering could be the basis of consciousness at the quantum level.
Anton (the narrator) does a great job on many scientific subjects and he is good at breaking down this complex subject, but you have to watch it till near the end. Have we lost our minds posting it to a Mineral and Geology Club? You can decide, but maybe consciousness is based on crystals after all.
Over 2-Billion Tons of Rare Earth Elements Found in Wyoming
From the Earth and I Article and Other Sources

Source: Wikipedia - Rare Earth Element Oxide Ores
The kinds of minerals discovered are widely used in technology, such as smartphones, hybrid cars, and aircraft as well as things like lightbulbs and lamps. These include oxides of neodymium, praseodymium, samarium, dysprosium and terbium.
Known for its natural beauty, the US state of Wyoming may soon be known for something buried beneath its stunning topography: An estimated 2.34 billion metric tons of rare earth minerals (REMs), which make the world’s computing-dependent technologies possible, were recently discovered near Wheatland, a town in southeastern Wyoming.
According to American Rare Earths, the company’s wholly-owned deposits have a potential volume far greater than China’s estimated 44 million metric tons of the minerals, which could establish the US as the world’s largest supplier. At present, China supplies about 95% of the global supply of REMs, 74% of which are imported by the US. If these deposits in Wyoming could be developed, it would change the balance of power and strategic reserves of REMs. According to American Rare Earths, the discovery has exceeded their expectations.
In a technical report issued earlier this month, American Rare Earths—the US division of a Sydney, Australia-registered exploration company—disclosed that it had discovered 64% more REMs than it had originally speculated in a March 2023 land assessment. Donald Schwartz, CEO of American Rare Earths, explained the surprise upgrade to Cowboy State Daily: “Typically, you’ll see the resource decrease as infill drilling takes place—instead we’re seeing the opposite, with only 25% of the project being drilled to this point.” Other mining companies have been exploring the area as well and are reporting REMs worth over 34 billion dollars in deposits near the surface. Sources are looking at mining these ores over the next 30 to 50 years, depending on the market and prices of Rare Earth Elements.
But don’t expect a sudden, dramatic increase in REM supply. Schwartz told Cowboy State Daily that the annual global demand for REMs is about 60,000 tons. “If you build a really big mine, can the market take all of that material?” he said. “We’re trying to make something that’s modular and scalable, that can grow in the market over time.”
The Rarest Diamond of Them All
From the Smithsonian Magazine Article - Full Credit to Kayla Randall
"It’s called the Winston Red Diamond, and what makes it so exceptional is the nature of its redness. Designated as a “fancy red” by the Gemological Institute of America (GIA), a global authority on gems, it’s one of the largest of its kind ever found. (The Moussaieff Red Diamond is the largest in the public record at 5.11 carats.) The stone takes its name from Ronald Winston, who gave it to the Smithsonian’s National Museum of Natural History in 2023, continuing a tradition that began with his father, Harry Winston, the famous jeweler known as the “King of Diamonds,” who donated the Hope Diamond to the Smithsonian in 1958," from the article.
In the simplest terms of understanding, diamonds are carbon minerals that were created over 3 billion years ago under extreme pressures deep in the earth. "Fancy" or colored diamonds are created by impurities or damage to the atomic matrix of the diamond's structure. To learn more about how color is created in diamonds, please watch the movie below.
Only about 30 percent of natural diamonds are considered gem-worthy, and among those, less than 1 percent are fancy color diamonds, that is, diamonds of unusual color. While the latter come in all hues, red seems to be the very rarest color of all.
Sources: https://www.youtube.com/watch?v=CwfR_jSZ9Fc&t=1s, https://www.smithsonianmag.com/smithsonian-institution/fancy-yourself-look-worlds-rarest-diamonds-red-smithsonian-natural-history-museum-180986244/
The Mohs Scale of Hardness
In 1812, the Mohs scale of mineral hardness was devised by the German mineralogist Frederich Mohs (1773-1839), who selected the ten minerals because they were common or readily available. The scale is not a linear scale and is based on 10 minerals in a ten-point scale that goes from one to ten. One (the softest mineral) is Talc, and 10 (the Hardest substance) is Diamond. It was created by one mineral's ability to scratch another softer mineral. While it is a very simple and straightforward way to test the hardness of minerals, it lacks some of the sophistication of later hardness scales and hardness tests used to measure specific materials like metals or wood. There are many graphics on the internet and even some on our other pages, but we wanted to expand and illuminate the much-used mineral scale to our visitors.
The Mohs Scale vs. the Vickers Hardness Scale with Mineral Examples:


When we see the Vickers hardness score, it gives us more information about the differences in hardness between the minerals that were used for the Mohs hardness scale, with some of the more notable takeaways being that talc to corundum (sapphire) is much closer in hardness than corundum is to diamond.
The main difference between the Mohs scale and the Vickers scale is that the Mohs scale measures hardness by comparing a material's ability to scratch another material on a relative scale from 1 to 10, while the Vickers scale uses a precise indentation test with a diamond pyramid to measure a material's absolute hardness.
This information is really useful, but the problem is outside of the results for the materials. To put it simply, the Vickers (or Knoop, which is similar) hardness tests aren’t really suitable for gemstones as they involve a small impact rather than a scratch, as with the Mohs test. Gemstones are generally brittle by nature, and they have a tendency to fracture when being tested, which isn’t ideal, which is why we still use the Mohs scale. In addition, it's not always feasible to carry bulky hardness testing equipment around when you're in the field.
The Mohs Scale of Hardness
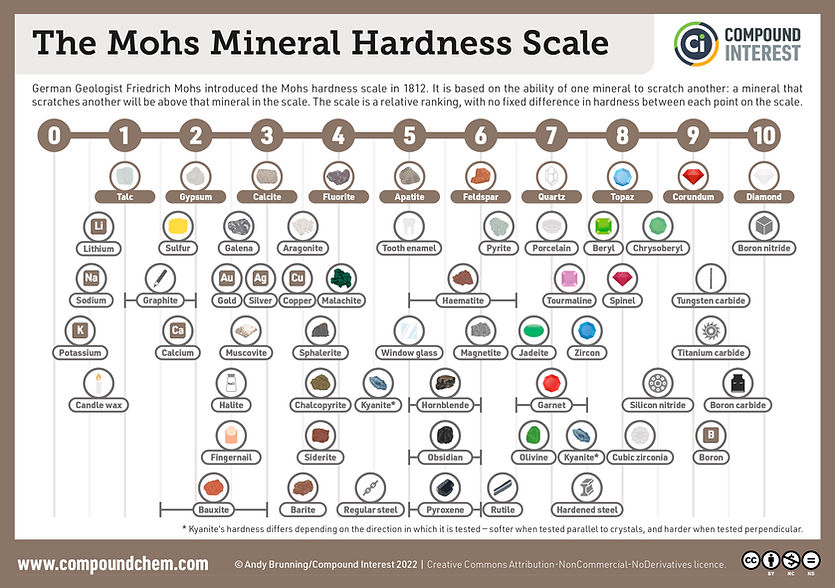
This handy downloadable chart is useful and is free to use as a reference.
Still Want More?
So if you are really interested in testing the hardness of minerals for industrial or identification purposes, there is a very handy kit that you can get that basically comes in something like a plastic drill bit box, and they are not too expensive. It's called a "Mohs Hardness Scratch Testing Kit" or sometimes a "Concrete and Stone Hardness Kit." But to be fair, it's only worth it if you are commonly finding yourself in a situation where you really need to identify minerals or the hardnesses of things on a pretty regular basis. These sell for around $130 USD, and we would recommend buying them from a reputable source, as cheap copies do exist.

New Fossil Evidence of Color, Protofeathers and
Vocalizations in Dinosaurs
In a very interesting video, the narrator lays out the case for color based on fossil evidence. Even the mechanisms of dinosaur vocalizations have been seen in supremely well-preserved ancient animals. This paints a picture of a much richer story than we might believe of the animals that lived millions of years ago, whose lives seem every bit as complex and fascinating as those of animals today.
This video is about a half-hour long, so grab some popcorn and a pop, and be prepared to be amazed at what we clever humans have been able to deduce from the remnants of long-dead denizens of the past.
How Earthquakes Build Gold Nuggets
When strained by earthquakes, underground networks of quartz veins can generate enough voltage to snatch gold from passing fluids, researchers report September 2 in Nature Geoscience. The findings explain how fluids carrying meager amounts of gold can concoct large nuggets, even in chemically inert settings.
An estimated 75 percent of all mined gold comes from deposits nestled in cracks inside hunks of quartz, one of the most abundant minerals in Earth’s crust. Geochemists have known that dissolved gold existed in fluids in the middle to lower levels of the planet’s crust and that the fluids could seep into quartz cracks. how these gold particles begin sticking to one another in a single location, eventually forming nuggets that can weigh up to hundreds of kilograms, has presented geologists with a puzzle.

Gold forming on Quartz matrix could be a piezoelectric effect from tremors and earthquakes. Electric flow in quartz veins could help gold particles clump into nuggets.
The new study, published in Nature Geoscience, suggests that the geological stress caused by earthquakes might activate a peculiar geochemical property called piezoelectricity—and that such activation makes the formation of larger gold nuggets possible.
The piezoelectric effect, which has been known since the 1880s, is essentially the ability of a material to generate an electric charge when placed under mechanical stress. Many everyday items including microphones, musical greeting cards and inkjet printers take advantage of piezoelectricity, and it occurs naturally in substances from cane sugar to bone.
Quartz can produce this effect because of its structure: it is built from a repeating pattern of positively charged silicon and negatively charged oxygen atoms. When it’s stretched or compressed, the arrangement of these atoms changes and the charges are dispersed asymmetrically. Negative and positive charges build up in different areas of the quartz, creating an electric field and changing the material’s electric state.
Voisey and his colleagues at Monash—located in the historically gold-rich area of Melbourne—thought that this changed state could lower the energy needed for gold nanoparticles in the fluid to interact with the quartz surface, causing a previously unviable chemical reaction to occur and allowing the nanoparticles to stick and accumulate.
To explore their idea, the researchers virtually modeled the electric field that quartz could produce when subjected to earthquake-like forces. They then placed quartz mineral crystals in a fluid containing dissolved gold nanoparticles and other gold compounds and found that, when under seismic wavelike forces, the quartz was able to produce enough voltage to jump-start a buildup of nanoparticles.
To test this, the team submerged quartz crystals in a solution containing tiny, free-floating gold particles. Then they quickly squeezed and released the quartz crystals over and over with a motor, mimicking the frequency of seismic waves. Voisey and his team found that even modest stress on the quartz led to gold accumulating on the crystal surfaces. Over time, they say, these accumulations would grow into larger and larger fragments.
Voisey and his team plan to extend experimental parameters by testing different pressures or temperatures, for example, to explore their theory further. “This is very much the pilot study for this technique,” he says, “so I’m excited to see where it can go.”
Sources: https://www.scientificamerican.com/article/earthquakes-may-forge-large-gold-nuggets/#:~:text=The%20new%20study%2C%20published%20in,of%20larger%20gold%20nuggets%20possible; https://www.sciencenews.org/article/earthquakes-build-gold-nuggets, Voisey, C.R., Tomkins, A.G. and Hunter, N.J., 2019. Gold Accumulations in Quartz Driven by Earthquake-Induced Piezoelectricity. Crustal-to Nano-Scale Influences on Orogenic Gold Deposits: Insights from the Central Victorian Goldfields, Nature Geoscience. Published online Spetembr 2, 2024; https://www.science.org/content/article/shake-rattle-and-gold-earthquakes-may-spark-gold-formation
Fossils and Geology of Michigan
Brought to us by our friends at the Michigan Geological Survey, here is an excellent primer on the geological ages and how the great state of Michigan formed all the wonderfulness that is the Great Lakes. The video features some really solid 3D visuals, fly-around, and timescale graphics behind the different sedimentary layers and their potential fossils that you can find in our state. It's a 7:12-minute video that will leave you wiser and in awe of Michigan's geological development.
Common Fluorescent Minerals: A Reference
Common Fluorescent Minerals: A Reference
Over 500 minerals have been discovered that exhibit some fluorescent behavior when exposed to ultraviolet light. Below is a handy guide to many of the more common minerals that you can download a PDF to keep. This handy guide from our friends at the Fluorescent Minerals Society website is a great quick guide to the common colors for minerals that exhibit fluorescence. It can also be very handy to use a UV light source for hunting fossils as well, as they often have calcite in th, which usually glows a pale to bright yellow color when illuminated with those handy UV flashlights. Remember to wear your eye protection when using strong UV sources!
Click to download this handy common Fluorescent Mineral Guide in PDF form. It displays over 20 minerals and mineraloids with thier common colors under Ultraviolet light.
Anomalocaris: Earth's First Predator
Over the next few months, we will focus on fossils and the life that created them. This video shows some of the earlier life that evolved on planet Earth and gives us a glimpse of the earliest times when life seemed very alien from today and seemingly large shrimp-like creatures ruled the oceans. During the early Cambrian age, it seems like life was a grand experiment, and we are left to piece together the fossil record.
The Colorful Salt Mines of Belarus

Multicolored walls of a salt mine located 1,380 feet (420 meters) underground, near the town of Soligorsk, south of Minsk, Belarus. Parts of this mine have been converted into a speleotherapy clinic for treatment of respiratory illnesses such as asthma and bronchitis.
Opened in 1949, this mine was notable for its salt, which containes high levels of potash (salts that contain water-soluble potassium). One of the five excavation chambers opened here in the last 50 years is still in use to collect minerals, but the rest have been transformed for halotherapy, or salt therapy. It is believed that the dry air charged with salt ions has a positive chemical effect on the respiratory system. Many patients who come here for treatment are children from the regions of affected by the Chernobyl disaster, but patients travel from countries like Russia, Japan, and Ukraine to receive treatments. The mine is only open to patients and staff in order to avoid disturbing the treatments.
Salt deposits in Soligorsk, are visible long before the entrance to the city. Salt mining in Soligorsk has been carried out since the 1950s on one of the largest deposits in Europe. Dumps of red color with dazzling white impregnations of salt create an extraterrestrial landscape, which is supplemented by mud sinks and artificial lakes. The wonderful colors are a natural occurrence caused by evaporation zones, sediments, and the mineral carnilite, which forms after a salty sea, dries up, leaving behind the different colors of the same mineral.
To visit this place you would need a special permit.

Photographer Viktor Lyagushkin ventured into Berezniki, Belarus's sylvinite mine to photograph the psychedelic underground world.
Sources: https://www.independent.co.uk/travel/belarus-salt-mines-healing-allergies-travel-a8932941.html, https://www.theatlantic.com/photo/2019/04/photos-strange-beauty-salt-mines/586417/, https://www.greatvaluevacations.com/travel-inspiration/worlds-most-beautiful-salt-mines, https://www.pinterest.com/pin/665406913705565233/
Did the Holy Grail of the modern world just get invented? Well, maybe not invented, but found by using a lead phosphate apatite mineral? Replace a few lead atoms with copper and viola! We wanted to get this in front of you becasue it seems very promising and if its true, it will change the world and transform how we use energy very soon. Not only does this change materails science and transform our energy hungry society, it could lead to other inventions that could quickly change the world!
Please watch this to the end; there's quite a bit of ground that it covers, and the explanations are well done, so it's worth a good watch. This little black rock floating on the magnet is an amazing scientific find. Once again, minerals have changed the world!
For our January story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. These two are very similar in name and often confuse collectors just by the name alone.
So what's it gonna be?
Stibnite vs Stilbite
While both minerals have very different appearances, remembering their names often trips people up. They are visually very different and do not share any common chemistry, nor are they found together.
Stibnite
Formula: Sb2S3 - An Antimony Sulfide

-
An important Antimony ore
-
Used in ancient times as an eyeliner.
-
It is named after the Latin name for the element Antimony.
-
Comes in around 2 on the Mols hardness scale.
-
Also known as antimony glance, antimonite, and stibine.
-
It has a gunmetal metallic luster, very much like Galena.
Stibnite occurs in hydrothermal deposits and is associated with realgar, orpiment, cinnabar, galena, pyrite, marcasite, arsenopyrite, cervantite, stibiconite, calcite, ankerite, barite and chalcedony. Small deposits of stibnite are common, but large deposits are rare. The world's largest deposit of antimony, the Xikuangshan mine, yields high-quality crystals in paragenesis with calcite. It occurs in Canada, Mexico, Peru, Japan, Germany, Romania, Italy, France, England, Algeria, and Kalimantan, Borneo. In the United States it is found in Arkansas, Idaho, Nevada, California, and Alaska.

Stibnite from Romania. Image provided by Wikipedia Commons.
Stilbite
Formula: NaCa4(Si27Al9)O27•28H20 - A Zeolite

-
As a Zeolite, Stilbite can be used in industrial filtering processes.
-
Named in 1797 after its glittery shine or "stilbe".
-
Comes in around 3.5 to 4 on the Mols hardness scale.
-
Its customary shape and nodules are often described as looking like cauliflower.
-
Its color can vary between white, creamy, light yellow, light grey, and pinks to orangy-red.
At one time heulandite and stilbite were considered to be identical minerals. After they were found to be two separate species, in 1818, the name desmine ("a bundle") was proposed for stilbite, and this name is still employed in Germany. Stilbite shows a wide variation in exchangeable cations. Since silicon and aluminium have a different charge (Si4+ and Al3+) the ions occupying the sodium/calcium site have to adjust to maintain charge balance. There is a continuous series between stilbite and stellerite, Stilbite crystals are typically thin tabular, flattened parallel to the dominant cleavage, and elongated along the a-axis. Aggregates may be sheaf-like or in bow-ties, also fibrous and globular. Twinning, cruciform, and penetration, is extremely common

Stibnite (yellow-orange color, with Fluorapophyllite (greenish color) on white or clear Stilbite from Maharashtra, India. Image provided by Wikipedia Commons.
Sources: https://www.mindat.org/min-7313.html, https://www.mindat.org/min-3782.html, https://en.wikipedia.org/wiki/Stibnite, https://en.wikipedia.org/wiki/Stilbite
An Amethyst story
Article Adapted from: https://flipboard.com/video/businessinsider/92b68af35f
Originally Written by Business Insider
How miners find, cut, and transport the most expensive amethysts in the world!
Amethyst is one of the most abundant crystals in the world. But the most prized pieces can cost almost a million dollars. Some of the world's largest amethyst geodes come out of Artigas, Uruguay. The earth beneath this region is uniquely suited to producing amethyst. But other than size, what qualities do miners look for in a valuable amethyst? And how are the crystals prepared once they're out of the ground? We explored why amethyst geodes are so expensive.
Ulexite
The Amazing Fiber Optic Mineral
Article Adapted from: https://rockseeker.com/ulexite/
Originally Written by Jeremy Hall
Ulexite is a strange, fibrous mineral with some interesting optical properties. It’s sometimes referred to as television stone, owing to the strange visual properties that it carries. It’s a complex matter and one that takes a bit of study to figure out. So, without further ado, let’s break in with our guide to ulexite, and see if we can’t shed some light on this fascinating mineral.

What is Ulexite?
Ulexite is a boron mineral, specifically hydrated sodium calcium borate hydroxide (NaCa[B5O6(OH)6] · 5H2O). It appears as bundles of transparent fibers tightly “bound” together. Boron is a very rare element, found in high concentrations in only a few places across the planet. It’s also used in a surprising amount of processes that are essential to our modern lifestyle. For instance, borosilicate glass is preferred over other types when heat or thermal shock may be an issue since it resists both of those quite well.
You probably have some borosilicate glass in the kitchen; it’s generally called Pyrex. Boron is never found in its elemental form in nature. Instead, it’s found in a wide range of different minerals. Many of these are of commercial importance, but the three main sources of boron for industrial use are ulexite, borax, and colemanite. Boron is also used to harden steel when added to alloys. Overall, it’s useful stuff even when it’s not of interest to mineral collectors. Ulexite is a standout option due to occurring in relatively large amounts when found and being easy to process in order to liberate the boron molecules for use. Ulexite is also being explored as a starting medium for sodium borohydride. This compound is thought to be an ideal storage mechanism for hydrogen fuel cells due to its high weight-to-hydrogen ratio.

Where Can You Find Ulexite?
Ulexite is most often found in evaporite deposits. Evaporite deposits are known to contain many boron minerals, from howlite to ulexite, and also contain things like halite, gypsum, and calcite. These minerals are all soluble in water to some extent. Essentially, these deposits were thrown from volcanic activity, termed pyroclastic, which contained boron. Water flowed over these stones and to lower ground, slowly removing minerals from the stones. These include boron minerals, such as borax and ulexite. Because of this, you’ll want to make sure that you don’t expose any ulexite samples you have to water. Hot water, in particular, will dissolve or damage your sample. If you do need to rinse dust or dirt off of a specimen, it’s best to use a rag that’s been made damp with cold water.
In general, you’ll find evaporite deposits in dry lakes. A dry lake is one that fills up with water during the rainy season but loses water quicker than it gains it. This results in the lower ground being encrusted with salts picked up from the pyroclasts. Over time, these can often form into a stratum. A stratum is a specific layer of stone, so an area with large amounts of boron will often end up with layers comprised entirely of borate beneath the surface.
The most prevalent source of the material is the Boron Pit in Boron, California. Unfortunately, you’re probably not going to be able to collect from this area. While there is an area farther down the mountain where the tailings are dumped, this is both not allowed and incredibly dangerous. The Boron Pit is actually the world’s largest borax mine. It’s a pit mine with an enormous circumference and 755 feet or so deep. However, the mineral isn’t hard to acquire. A single individual, David Eyre of Desert Discoveries has an exclusive contract to collect the optical grade ulexite which is removed from the mines owned by Rio Tinto. Fortunately, optical ulexite is widely available online if you really want to own a piece of this natural oddity.

Photo Credit: Minerals.net

Ulexite Slab from Mindat.org
The "TV Stone"
Ulexite is often called the “TV Stone” due to its strange optical properties. The fibrous form of the mineral transmits light somewhat like a fiber optic cable. When cut across these fibers, it can transmit an image clearly. This is incredibly rare with natural minerals, the only other one known to do so is fibrous selenite. Selenite, however, does not transmit nearly as clear of an image. Keep in mind that this is different than the mineral simply being transparent, instead, the image is actually projected onto the surface of the ulexite. The internals of these fibers are perfectly reflective. The fibers themselves are about 2 micrometers across. This only works when the fibers are parallel to the image you’re trying to project. If you rotate the stone 90 degrees it no longer displays the properties. Large samples of ulexite in this form are vanishingly rare. The problem is the same as the reason it forms. Quite often you’ll also find mud or other inclusions that were trapped during the formation of the mineral. These can obscure the view and render it more fit for industrial use than demonstrating its natural fiber optic qualities. Pieces a few inches across are common finds on any site that sells minerals, and for many people, this may be the only way to collect it.
"Bunny Tail" Ulexite
Ulexite is sometimes found in a form that resembles a cotton ball. You’ll also see this form referred to as “bunny tails” in some places. These are actually comprised of small, needle-like crystals. While an interesting specimen, people are generally more interested in finding the larger masses of fibrous crystals since the tufts can’t be worked into a form that shows off the unusual characteristics of the mineral. That said, the acicular form can make for incredible specimens. This form is very fragile, however, and care must be taken when searching for it. They generally grow in hollows in the bedrock. The acicular formations may be found singly, or in overlapping patterns. Some of these can be quite dramatic in effect. Unfortunately, this form is also hard to find for sale since there’s not much demand for it.

The Amazing World of Trilobites
A Brief History of Trilobite Shapes and Ecology...
Adapted from "The Colorful World of Trilobites" by Pete Buchholz and Franz Anthony
The fossils of small-segmented animals had been known to humans for centuries, but for most of that time, no one was really sure what they actually were. Their durable armored exoskeleton and habit of molting ensured that they were easily fossilized and discovered by people hundreds of millions of years later. Some Native American people used Elrathia fossils as amulets and back in 1679 the first scientist to study Ogygiocarella thought it was a flatfish skeleton.
Trilobite fossils are far more than small bumpy black fossils that scuttled around on the ocean floor. They can be found all through the ancient oceans of the earth and even ventured on land near the beaches. They evolved their own unique excellent eyesight that they used to find food and even hunt. It's very likely that they came in a host of colors like today's crustaceans.
Throughout the 18th century, many new fossil species were discovered, confounding scientists about their identities. Their segmented bodies and compound eyes reminded scientists of insects and crustaceans, offering clues to their true identity. By the dawn of the 19th century, these fossils, now known as trilobites, were considered to be quite like crustaceans, but not exactly. They were unique among known animals with no living examples.

Image (Left to Right): Fallotaspis, one of the oldest trilobites from the group Redlichiida, the 'stealth bomber' Lonchodomas, and the upside-down Carolinites. Image by Franz Anthony.
Although they have a lot of anatomical diversity, trilobites all follow the same basic body plan. They’re divided into three lobes; the left, right, and center. They also have three main body sections, a head, several torso segments, and a tail, with some having a long tail spine. These features make them easy to identify for amateur fossil hunters.
Trilobite Diversity
As the Paleozoic progressed trilobites pushed their limits, moving into new environments. Trace fossils of tunnels, called Cruziana, were formed by trilobites digging under the surface. There are footprints of trilobites walking on the sandy upper beach of an ancient shore at low tide. They were among the first animals to ever venture out of the seas, even if it was just for a few minutes. Some species ventured into the deep sea, becoming completely blind where daylight never penetrates.
They are famously segmented and are part of a huge lineage of animals with segmented legs and bodies known as arthropods. That lineage also includes insects, crustaceans, millipedes, spiders, scorpions, and many others. Trilobites may look a lot like some living arthropods like horseshoe crabs or pill bugs, but they are only distantly related to them, and were one of the earliest arthropod groups to emerge.
In their time, the humble trilobites filled roles today occupied by crustaceans. They didn’t just fill in for the crabs scuttling along the bottom, but shrimp swimming in the water and digging in the sand, and even filling the roles of many living fish like catfish and small sharks.
Armored Shells
The fossils we find are normally just the upper halves of trilobites. Their upper parts were strengthened with calcite like the shells of crustaceans, but their legs and bellies were armored only with stiff proteins that usually decomposed before fossilizing. Many trilobites were covered in defensive spines and barbs as a way to protect themselves from being eaten. Others, like Phacops, evolved a defense mechanism later copied by pill bugs and armadillos: rolling into a ball. Many specimens of enrolled trilobites record their final fight for survival.
Calcite Eyes
The earliest trilobite eyes are known as holochroal and had densely packed lenses, with a single corneal layer covering the whole eye. The vast majority of trilobites have holochroal eyes, but not all of them. A lineage of trilobites known as the phacopids evolved a modified type of compound eye called schizochroal, where each calcite lens is separated from its neighbors by exoskeletal tissue and each has an individual cornea. Schizochroal eyes resemble the eyes of young trilobites with holochroal eyes that may have been the result of paedomorphosis, the retention of juvenile features in adult animals. Some trilobites grew eye stalks so they could see 360 degrees in every direction, or even used their eyes like a periscope while buried under the sand.
Diet and Food
Trilobites had mouths on the bottom of their heads and like their insect and crustacean cousins had numerous complex mouthparts to help them eat. Like insects and crustaceans, they also had diverse diets. Some ate algae, while others, like Scotoharpes and kin, may have been specialized filter feeders. Many ate dung and detritus while others were carnivores, some even feeding on smaller trilobites.
Antennae, Legs & Colors
The highly mobile, visually oriented, and social animals came in all kinds of shapes and sizes, behaviors, and surely colors and color patterns. Their rarely fossilized antennae and legs emerged from under their shield-like shells, and allowed them to move, interact, and behave like fully competent creatures. Rare specimens tell the rest of the story, with curving antennae emerging from under the head, and one set of legs and gills for each torso segment.
Specimens of a trilobite called Eldredgeops even had freckles of calcite crystals on their back, rare evidence of a color pattern that the animal sported in life. The spots might have been used as a form of camouflage, blending in with the sandy bottoms to avoid predators.

A selection of trilobites, illustrated in bright colors inspired by today's crustaceans.
Image (Left to Right): Triarthrus, the first trilobite known with legs and even eggs preserved; Miraspis, a spiky trilobite with eyestalks; and Eldredgeops, a trilobite with calcite crystal spots on its back. Image by Franz Anthony.
Trilobite Fossils and Illustrations Today
Some spectacular trilobites were fossilized with pyrite, also known as “fool’s gold.” They are the brilliant metallic reflections of a lost world. Most trilobite fossils, however, are not golden, but instead black or brown. Perhaps because of this, most illustrations of living trilobites show them as black or brown inverted boot prints. This isn’t necessarily sound reasoning, because the color of fossils is largely based on their mineral content. Fossil crustaceans like crabs and lobsters are often black and brown too, but we know from living species that they’re almost never solid black, but come in all manner of colors and patterns.
Although trilobites are common fossils, they weren’t found in every rock those early “gentleman scientists” looked at. Like all fossils, trilobites are only found in sedimentary rocks, but it’s more specific than that. Trilobites were strictly marine and lived only in the Paleozoic Era, 541-252 million years ago.
Their Extinction
If we were to travel back in time to the seas of the Paleozoic Era, we’d meet our heroes on familiar reefs and intertidal beaches, where they’d be joined by a largely unfamiliar cast of characters. “Forests” of stemmed starfish relatives called crinoids, lived just below the waves, and the seas were full of shelled relatives of squid and octopi. The reefs themselves were built by encrusting algae and completely extinct types of coral.
And on those reefs were hundreds of diverse species of trilobites; crawling, digging, swimming; eating, hiding, raising young; living and dying. Earth of the distant past wasn’t inhabited by aliens doing alien things, but by normal animals doing wonderful things.
Through the nearly 300 million years of the Paleozoic Era, trilobites were hit by multiple mass extinctions, and in one case they were almost wiped out with only one genus making it through. They finally fell victim to extinction 252 million years ago at the end of the Permian Period, in a mass extinction commonly called the Great Dying. This extinction is linked to huge volcanoes in Siberia whose eruptions burnt vast coalfields. The CO2 dumped into the atmosphere from the volcanoes, and especially the coal, led to rapid global warming and ocean acidification. This proved too much for the trilobites to survive and they, as well as 90% of marine animal species, disappeared.
References: Special thanks are owed to paleontologist Dave Rudkin of the Royal Ontario Museum in research and preparation of the illustration. Andy Secher, Martin Shugar. “Trilobite Website.” American Museum of Natural History. Accessed 27 May 2018. Cardiff Curator (@CardiffCurator). “U is for Utah, USA. Native Americans in this area used Elrathia trilobites as amulets to protect against disease and injury #TrilobiteTuesday #Alphabet” 27 Feb 2018, 12:15 AM. Tweet. Jane J. Lee. 2013. “Trilobites Found With Mysterious Markings.” National Geographic News. Accessed 10 April 2018. Markus Martin (@trilobitelegs). “Pregnant for nearly 450 million years...A golden Triarthrus eatoni with soft tissue preservation and eggs. Yay for trilobite eggs!” 20 Feb 2018,7:03 AM. Tweet. M. Gabriela Mángano, Luis A. Buatois, Ricardo Astini, Andrew K. Rindsberg. 2014. “Trilobites in early Cambrian tidal flats and the landward expansion of the Cambrian explosion.” Geology; 42 (2): 143–146. Raymond C. Moore. 1959. “Treatise on Invertebrate Paleontology Part O, Arthropoda 1.” Geological Society of America, University of Kansas Press. Richard Fortey. 2001. “Trilobite: Eyewitness to Evolution.” Vintage. Richard Fortey. 2004. “The Lifestyles of the Trilobites.” American Scientist, Volume 92. Sam Gon III. “A Guide to the Orders of Trilobites.” Accessed 27 May 2018.
The Confusing term "ONYX"
What does it mean and why can it look so different?
From Wikipedia:
Onyx primarily refers to the parallel banded variety of chalcedony, a silicate mineral (Quartz). Agate and onyx are both varieties of layered chalcedony that differ only in the form of the bands: agate has curved bands and onyx has parallel bands. The colors of its bands range from black to almost every color. Commonly, specimens of onyx contain bands of black and/or white. Onyx, as a descriptive term, has also been applied to parallel banded varieties of alabaster, marble, calcite, obsidian and opal, and misleadingly to materials with contorted banding, such as "Cave Onyx" and "Mexican Onyx".
As stated, onyx is both a banded chalcedony of very good to excellent quality for carving and jewelry that is a quartz mineral. Or it is a colored banded calcite or banded stone visually related to banded calcite. There are even websites that will argue that there are additional differences between Banded Agate (chalcedony) and true Onyx. So how do you tell? Well, that's why we're here today!

Back and white Onyx cabochons, some with translucent banding.

Red-Orange and white banded Carnelian (Sardonyx) cabochons.
To Onyx, or not to Onyx... That is the Question!
In many ways, probably 90% of the time, it's really easy to tell if you're getting Onyx or something that is just traded under the name of Onyx. If it's a deep black color or banded blacks and white, mostly opaque, and has a polished shiny glass-like or vitreous luster, it's probably Onyx. Onyx like this is usually carved and polished to be cabochons for wearing in jewelry.
If it's rusty red or orangy-red and white banded material and is hard and shiny like glass, it's probably what we call "Sardonyx" or "sard" or even "Red Onyx".This is the same thing as Onyx (banded chalcedony or Quartz) but is red-orangy in color with white bands. They might also call this Carnelian; which is really the same thing; we're just splitting hairs. Please note the examples.
If it's not the above, and instead has a waxy luster and seems like you might be able to scratch it with your fingernails, its colorful with different bands running through it, and it's NOT black, you probably have banded calcite that's being called Onyx under the trade name. The hardness for calcite is around 3, and your fingernail (untreated) is about 2.5, so it kind of feels like you can scratch or mar it a bit just by running your fingernail along it.
Chalcedony and Quartz have a hardness of 6.5 to 7, so they will feel glassy hard and not as easy to scratch. It also takes a polish better than calcites and alabaster and many other materials sold under the trade name of "Onyx". The material very often looks shinier and smoother, but not always.

Banded Calcite, Marble, and Alabaster Examples
If it's not black and white and seems banded with many colorful layers, and feels a bit soft in your hand, it's probably calcite. There are many places in the world where calcite is mined and distributed under the trade name of "Onyx". Places like Iran, Mexico, Arizona, Morroco, all over. In addition to banded calcite being sold as "Onyx", there is also marble, alabaster, and other banded translucent stone sold under the same "Onyx" trade name. Onyx is really just a generic term that does not have any real meaning anymore other than it's popularly known. Here are a few examples that you can visually scan. Some are Mexican calcite, some are Alabaster, some are marble or cultured granite, and yet they are all sold as Onyx!
We would forewarn you that countertop places love to just call things what they want, just like paint colors have all kinds of crazy names, so make sure that when you're looking for countertops, it's not calcite. It's pretty but too soft for everyday living.
The Acid Test
Lastly, if you really gotta know, sometimes a bit of white vinegar can do the trick. The acid in the vinegar will eat at the calcite, and it will bubble or foam a bit, but only do that with permission and in a place that doesn't show. Vinegar will not harm Quartz or Chalcedony.
The Above Example: Swatches of banded calcite, alabaster, marble, granite, and many cultured stone products are sold under the name of "Onyx". Can you tell which is which? Well, none of them are actually the Quartz variety of true onyx.
Pink & Rose QUARTZ
Can you tell the difference just by looking at it?
All Credit to Steve Voynick of Rock and Gem Magazine (Feb. 2023) for this Article
Rose quartz ranks high among the most attractive and familiar of the many color varieties of quartz. Its delicate pink color, soft translucency, and affordability make it a popular gemstone. Pink quartz is a much lesser-known variety of quartz. While similar to rose quartz in color, it differs markedly in structure, degree of transparency, the origin of color, occurrence, rarity, and cost.
Pink QUARTZ
Pink quartz, a pink macrocrystalline variety of quartz, was discovered in pegmatites at Rumford, Maine, and first described in mineralogical journals in 1938. But these specimens attracted little attention from mineralogists or collectors at the time. Initially, they were assumed to be a rare, atypical subvariety of rose quartz.
Then in 1959, pegmatite miners in Brazil’s gemstone-rich Minas Gerais state discovered clusters of beautifully developed, terminated, hexagonal quartz prisms. These crystals had water-clear transparency and a pink color that was similar, but not identical to, the color of rose quartz. When these specimens appeared on the collector markets of Europe and the United States, the limited supply was snapped up by both collectors and mineralogists.
Mineralogists soon learned that the color of pink quartz, unlike that of rose quartz, is created when some silicon ions within the quartz crystal lattice are replaced by trivalent aluminum ions and pentavalent phosphorus ions. This partial replacement renders the lattice susceptible to distortion from the energy of natural geophysical radiation, creating color centers that form when radiation displaces phosphorus ions from their normal lattice positions, leaving voids that trap electrons. When white light boosts these trapped electrons to higher energy levels, they return to their normal levels by releasing excess energy as visible light that we perceive as pink or pale red.

Pink Quartz crystals surround the center of golden Citrine Quartz. Photo credit to Wikimedia Commons.

Pink Quartz Specimen from Brazil. Photo credit to Psycodelic Rocks/IG

Raw Rose Quartz. Image Credit to Cape Code Crystals.

Rose QUARTZ
Rose quartz always occurs in massive form without crystal faces or terminations. It is almost always translucent, with a uniform color distribution, and is found mainly in the core zones of granite pegmatites.
Mineralogists had traditionally attributed the color of rose quartz to traces of titanium and, to a lesser extent, iron and manganese. These impurities were thought to distort the crystal lattice, causing it to reflect and transmit red wavelengths of light which the human eye perceives as varying shades of pink.
More recent studies have shown the pink color is because of fibrous inclusions. After dissolving rose quartz from several different sources in hydrofluoric acid, researchers have recovered residues of flaky, pink-colored nanofibers, most consisting of dumortierite and other aluminum borosilicates.
Although these nanofibers make up only about one-tenth of one percent of the overall weight of rose quartz, they are highly reflective and create both its characteristic pink color and its soft translucency. Usually aligned along the axes of quartz’s hexagonal crystals, these inclusions also explain the six-rayed asterism that appears in the star variety of rose quartz.
Star Rose quartz cut spheres showing the asterism of fibrous inclusions along the hexagonal axis. Photo credit to Martin P. Steinbach.
The differences
The inclusions in massive rose quartz inhibit crystal development, while the absence of inclusions in pink quartz ensures normal crystal development and a high degree of transparency. In addition, the color of pink quartz is often zoned and most intense near the crystal terminations. The color of crystalline pink quartz also fades slowly with prolonged exposure to sunlight, while the color of massive rose quartz is stable. And pink quartz is sometimes intermixed with citrine, the golden-yellow color variety of quartz.
The formation of pink quartz requires unusual and complex chemical and physical conditions that include partial ionic replacement within the quartz lattice, sufficient geophysical radiation to create color centers and enough space to permit crystal growth. Because these conditions don’t often occur together, pink quartz, unlike rose quartz, is rare, costly and found in only a few localities. A fine specimen of pink quartz can cost hundreds of dollars.
Another difference between the rose and pink subvarieties is size. Rose quartz often occurs in large masses; pink quartz is found only as crystals an inch or two in size. Given these collective differences, the term pink quartz is now used to differentiate crystalline pink quartz from massive rose quartz.
In general usage, the descriptive color terms pink and rose are often imprecise. Rose quartz is sometimes sold as pink quartz and vice versa. Also, massive rose quartz is frequently cut into hexagonal prisms for use in pendants; while these may appear to be natural crystals of pink quartz, their translucency immediately identifies them as rose quartz. Pink quartz is almost always retained as a specimen in its natural form.
Sources: https://www.rockngem.com/can-metal-detectors-detect-rocks/, https://www.geologyin.com/2018/07/what-causes-pink-color-of-rose-quartz.html, https://capecodcrystals.com/pages/rose-quartz-meaning
Scorodite
This Mineral is all about the Blues
Named for the Greek scorodion, meaning “garlic,” in reference to the garlicky smell Scoodite produces when heated. From hundreds of occurrences, usually in small amounts, including Germany, the Czech Republic, Austria, England, Algeria, particularly large crystals from Namibia, Brazil, Mexico, the USA, Japan, and Australia. Scorodite forms as a secondary mineral from the oxidation of arsenic-rich sulfides. Scorodite is an oxide mineral of iron and arsenic with the composition FeAsO4•2H2O. Scorodite weathers to limonite. Scorodite was discovered in the Schwarzenberg, Saxony district, Erzgebirge, Saxony, Germany. Scorodite follows the orthorhombic crystal structure and has a Mol's hardness of 3.5 to 4.
Scorodite can be a beautiful crystal can be easily confused with euclase and even tanzanite and celestine. Its crystals can appear similar to all these, and its color can mimic these minerals as well. Transparent and clean crystals of scorodite demand a strong price from collectors and can fetch prices higher than many of the minerals it appears like.
Why is scorodite about the blues, you might be asking? Well, for its obvious blue coloration and its ability to easily fool a rock hunter into thinking he has found copper, sapphires, blue euclase, or even an out-of-place tanzanite vein. But its beautiful azure crystals can still fetch a fine price!

Unique Scorodite from Namibia. Credit: Key Minerals

Locality: Hezhou Prefecture, Guangxi Zhuang Autonomous Region, China

Scorodite, Namibia.

Scorodite from Ojuela mine, Mapimi, Durango, Mexico

Scorodite. Tsumeb Mine, Tsumeb, Oshikoto Region, Namibia. Credit: Heritage Auctions
Sources: https://en.wikipedia.org/wiki/Scorodite, http://webmineral.com/data/Scorodite.shtml#.Y9blqC-B03g, https://www.mindat.org/min-3595.html
CUPRITE
A Beautiful and Geometric Copper Mineral

Stunning Cuprite Crystals. Photo by: Laurent Kbaier

Red Dome Mine, Chillagoe, Queensland, Australia by Joe Budd
Cuprite is named for the Latin cuprum, "copper," in allusion to its copper content. Its chemical formula is Cu2O. It can form as bright transparent red crystals or as lustrous, submetallic opaque crystals. Even the opaque form will have slightly red edges and faint transparency upon back-lighting. Cuprite is often associated together with Native Copper in copper deposits and frequently forms as an encrusting reddish coating over the Copper. Malachite is known to fully or partially coat a layer or pseudomorph over Cuprite, forming an interestingly shaped and sometimes sparkling green crystal form.
Cuprite usually forms in octahedral crystals or in groups of octahedral crystals, sometimes with modified cubic crystal edges. it forms less commonly in cubic or in cubic clusters. Rarely in dodecahedral or modified dodecahedral forms and sometimes twinned as penetration twins, and occasionally in hopper growths.

Cuprite is commonly found as an oxidation product of copper sulfides in the upper zones of veins, often associated with Native Copper, Malachite, Azurite, Limonite, and Chalcocite. A fibrous form of Cuprite is known as Chalcotrichite. In rare or perfect conditions, it forms beautiful transparent bright red gem-quality crystals, but most of the time, the crystals are opaque or nearly opaque. In spite of its nice color, it is rarely used for jewelry because of its low Mohs hardness of 3.5 to 4. Faceted cuprite of any size is considered one of the most collectible and spectacular gems in existence, with its deep garnet coloring and higher brilliance than a diamond. Only the gem's soft nature prevents it from being among the most valuable jewelry stones.



Sources: https://www.minerals.net/mineral/cuprite.aspx, https://www.mindat.org/min-1172.html, https://www.geologyin.com/2017/05/stunning-cuprite-crystals.html, https://en.wikipedia.org/wiki/Cuprite
For the December story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. These two are very similar, in name, chemistry and how they can look, but they are distinctly different minerals.
So what's it gonna be?
Bornite vs Bournonite
While these two minerals share a similar locations and they are from the sulfides mega-group of minerals. Both these have a metallic luster, contain copper and can have bluish tarnish. Here is how they break down.
Bornite
Formula: Cu5FeS4 - A Copper Ferric Sulfide

-
An important Copper ore
-
Fresh surfaces can tarnish to various iridescent shades of blues to purples.
-
It is named after Hungarian Mineralogist Ignaz Von Born.
-
Comes in around 3 to 3.25 on the Mols hardness scale.
-
Often called Peacock Ore, Sometimes called Bornite.
-
Its color is caused by oxygen tarnishing.
Bournonite
Formula: PbCuSbS3 - Lead Copper Antimony Sulfosalt

-
An important metal ore.
-
A steely grey color with a metallic luster that sometimes tarnishes.
-
Named in 1805 in honor of Jacques-Louis, Comte de Bournon.
-
Comes in around 2.5 to 3 on the Mols hardness scale.
-
Often confused with, and found with other sulfosalts.
-
Its color can be caused by oxygen tarnishing.
Bornite, also known as peacock ore, is a sulfide mineral with chemical composition Cu5FeS4 that crystallizes in the orthorhombic system (pseudo-cubic). It occurs globally in copper ores with notable crystal localities in Butte, Montana and at Bristol, Connecticut in the U.S. It is also collected from the Carn Brea mine, Illogan, and elsewhere in Cornwall, England. Large crystals are found from the Frossnitz Alps, eastern Tirol, Austria; the Mangula mine, Lomagundi district, Zimbabwe; from the N'ouva mine, Talate, Morocco, the West Coast of Tasmania and in Dzhezkazgan, Kazakhstan. There are also traces of it found amongst the hematite in the Pilbara region of Western Australia.
Bournonite is a sulfosalt mineral species, trithioantimoniate of lead and copper with the formula PbCuSbS3. The crystals are orthorhombic, and are generally tabular in habit owing to the predominance of the basal pinacoid; numerous smooth bright faces are often developed on the edges and corners of the crystals. They are usually twinned, the twin-plane being a face of the prism (m); the angle between the faces of this prism being nearly a right angle (86° 20′), the twinning gives rise to cruciform groups and when it is often repeated the group has the appearance of a cog wheel, hence the name Rãdelerz (wheel-ore) of the Kapnik miners. The repeated twinning gives rise to twin-lamellae, which may be detected on the fractured surfaces, even of the massive material.

Bornite from Montana, USA. (public display, Montana Bureau of Mines and Geology Mineral Museum, Butte, Montana, USA)

Bournonite on Quartz: Yaogangxian Mine, Yizhang County, Chenzhou Prefecture, Hunan Province, China
Sources: https://en.wikipedia.org/wiki/Bornite, https://en.wikipedia.org/wiki/Bournonite, https://editor.wix.com/html/editor/web/renderer/edit/1b2f2ac4-5095-460f-82e7-a2c7a107f494?metaSiteId=4d698703-efef-4b33-8340-b641c265b39d
Lazaraskeite
A First Discovered Mineral that is Organic, Carbonate and Glycolate
Congratulations to University of Arizona Geoscientist Dr. Hexiong Yang and his colleagues for the discovery of a new mineral named Lazaraskeite, which has been published in the latest issue of “American Mineralogist, Vol 107, p 509-516, 2022”. Lazaraskeite represents the first organic mineral that contains glycolate. Its discovery implies that more glycolate minerals may be found and suggests that glycolate minerals may serve as a potential storage for biologically fixed carbon. It was found in the Western end of Pusch Ridge in the Santa Catalina Mountains, north of Tucson, Pima County, Arizona, USA. In order to be declared a new mineral, it has to be a naturally occurring crystalline substance, so man-made or industrial versions of the copper-glycolate substance don't count.
It resembles pale cyan blue to blue prismatic or bladed crystals, which many copper compounds exhibit, like veszelyite, clinoclase, and chalcanthite. The identification of this mineral is confirmed by single-crystal X-ray diffraction and chemical analysis.
Named in honor of Warren Lazar, an American prospector who discovered the mineral, and his wife Beverly Raskin Ross. They provided the first specimens for study. It has a chemical formula of Cu(C2H3O3)2. As any organic compound, the formula may also be written as Cu(OCOCH2OH)2 or Cu[O(CO)CH2(OH)]2. Associated minerals include chrysocolla, malachite, wulfenite, mimetite, hydroxylpyromorphite, hematite, microcline, muscovite, and quartz. Both polytypes are greenish-blue in transmitted light, transparent with a white streak, and have a vitreous luster. This crystal is brittle, has a Mohs hardness of ~2, and follows the monoclinic system.
Sources: https://pubs.geoscienceworld.org/msa/ammin/article-abstract/107/3/509/612025/Lazaraskeite-Cu-C2H3O3-2-the-first-organic-mineral, https://www.mindat.org/min-53400.html, https://www.geo.arizona.edu/news/2022/04/lazaraskeite-brand-new-mineral-first-organic-mineral-contains-glycolate-discovered-our, https://rruff.info/lazaraskeite/R180026



For the September story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. These two have caused a fair amount of confusion over the years as they can be visually very difficult to tell apart. They are both often used in jewelry making and carved into cabochons.
So what's it gonna be?
Variscite vs Turquoise
While these two minerals share a similar color but they are from the same phosphates mega-group of minerals. Both these sought-after gemstones are members of the Aluminum Phosphates group and both are greenish to blue-green in coloration. Here is how they break down.
Veriscite
Formula: AlPO4 · 2(H2O)

-
A rare to find hydrated Aluminum Phosphate.
-
Found in Aluminum rich rocks near the surface.
-
A vivid greenish to other colored phosphate.
-
It is named for the German location it was first discovered.
-
Comes in at 3.5 to 4.5 on the Mols hardness scale.
-
Sometimes called Verdite, Veriquiose, or Utahite.
-
The green color is from chromium impurities.
Variscite is a secondary mineral formed by direct deposition from phosphate-bearing water that has reacted with aluminum-rich rocks in a near-surface environment. It occurs as fine-grained masses in nodules, cavity fillings, and crusts. Variscite often contains white veins of the calcium aluminum phosphate mineral crandallite.
It was first described in 1837 and named for the locality of Variscia, the historical name of the Vogtland, in Germany. At one time, variscite was called Utahlite. At times, materials that may be turquoise or may be variscite have been marketed as "variquoise". Appreciation of the color ranges typically found in variscite have made it a popular gem in recent years. Varisite can have quite a bit of color variability from blue-green to pale green with veining remarkably similar to southwestern turquoise.

Variscite slab from Fairfield, Utah measuring 20cm.

Variscite cabochon from Gem Select.
Turquoise
Formula: CuAl6(PO4)4(OH)8 · 4(H2O)

-
A rare to find hydrated Copper Aluminum Phosphate.
-
Found in Copper-rich regions.
-
Named from the Turkish region that it was brought to Europe originally.
-
Comes in at 5 to 6 on the Mols hardness scale.
-
It is known by many names from all over the world.
-
The green color is from chromium impurities.
Turquoise is an opaque, blue-to-green mineral that is a hydrated phosphate of copper and aluminum. It is rare and valuable in finer grades and has been prized as a gemstone and ornamental stone for thousands of years owing to its unique hue. Like most other opaque gems, turquoise has been devalued by the introduction of treatments, imitations, and synthetics into the market. The robin’s egg blue or sky-blue color of the Persian turquoise mined near the modern city of Nishapur in Iran has been used as a guiding reference for evaluating turquoise quality. The color of turquoise and the veining can drastically affect its value.
The gemstone has been known by many names. Pliny the Elder referred to the mineral as Callais (from Ancient Greek κάλαϊς) and the Aztecs knew it as chalchihuitl.


Turquoise slabs cut from the original nodules and Turquoise
cabochons.
What Makes a Mineral vs. a Gemstone vs. a Crystal
After Many months of writing about minerals and crystals and gemstones, it was brought to our attention that perhaps not everyone may be in the know about why makes a differnce in these terms. They are not interchangeable and they do have specific meanings that distinguish them from each other. Sometimes the distinction can be important, especially if you're thinking about buying a gemstone, there are some question that are good to ask.
What is a Mineral?
The definition of a minerals contains a list of criteria that firmly define what a mineral can be, and what it not. See the example animations.
-
It occurs naturally - It can be found in nature and is not a man-made substance.
-
It cannot contain organic molecules.
-
It is a solid in its environment, because of the next statement.
-
It has an ordered atomic or chemical structure that repeats itself in a predictable pattern. In other words, the atoms that make it up can be predicted in name and spacial configuration.

Section of a Mineral Molecule
What is a Crystal?
The definition of a crystal contains a list of criteria that firmly define what a crystal is, and its definition includes more options than a mineral. Its criteria is a bit wider.
-
It occurs naturally or can be a man made substance. Synthetically created diamonds and rubies mimic a natural mineral, but because they are man-made they cannot be classified as a mineral.
-
It is a solid in its environment.
-
It can be inorganic, or organic in chemistry.
-
It has an ordered chemical structure that repeats itself in a predictable pattern.

Section of DNA Molecule
What is a Gemstone?
The definition of a gemstone takes a different tact. It loosely describes what a gemstone should be, but to be honest, a gemstone can be many things that provide focus to a piece of jewelry.
-
Can be a precious or semi-precious stone. (A stone is non-metallic earth or mineral matter hardened together in a mass.)
-
A gemstone does not need to be a mineral or a crystal. Opals and amber are considered gemstones, but they don't meet the strict definition of either state. Even glass and coral or an ammonite fossil can be a gemstone, but its not a crystal or mineral.
-
Being rare, beautiful to the beholder, and a fairly hard substance can definately help with longevity of a jewelry piece, and are all good qualifications, but not required.

Ammonite (Fossil-Opalized) Gemstone
Some Additional Context: In the three definitions, each one is unique. Its good to remember that these terms are often used incorrectly and sometimes interchangeably. By definition, a mineral has to be a crystal, but a crystal can be things that a mineral is not. Frozen water (ice) is a mineral, but sugar is a crystal since it contains organic chemistry. Fossils and opals can be considered gemstones, but they are not minerals, Crystalline substances like synthetic emerald can be a gemstones and a crystal, but are held to a different standard than naturally found gemstones. Even glass can be considered a gemstone, but it's not a mineral or a crystal, it merely serves as the focus of a jewelry piece. If your buying from someone you are not familiar with, best to ask what it is, and possibly what it is not, before you buy.
For the July story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. These two have caused a fair amount of consternation among mineralogists and collectors alike. They are, in a way, the same thing, but how the impurities within the mineral's structure organize themselves, makes all the difference.
So what's it gonna be?
Gem Silica vs Chrysocolla
While these two minerals share the same color but they are from the same silicates mega-group of minerals. Here is how they break down. They are so similar that some people don't even think that they are different at all, but its the hardness and gem quality that sets them apart.
Gem Silica
Formula: SiO2 Chalcedony with Chrysocolla inclusions

-
Very rare to find and is always found with copper deposits.
-
A vivid blue-green to turquoise colored variety of Chalcedony.
-
It is named for Silica Quartz family name.
-
Comes in around 6.5 to 7 on the Mols hardness scale.
-
Sometimes called Gem Chrysocolla, Chrysocolla Silica and Chrysocolla Chalcedony.
-
It is a Chalcedony colored by the same copper salts in the mineral Chrysocolla.
Gem Silica is a Chalcedony Quartz. Chalcedony is a form of Micro-crystalline Quartz (the crystals are so small that you can't see them with the human eye). This is why Gem Silica has a hardness around 7, since it is quartz with small amounts of Chrysocolla impurities that are spread within the Chalcedony. The Chrysocolla gives the silica its beautiful and vivid blue-green coloration.
Because Gem Silica is extremely rare to find, and it's a collector's gemstone, prices can be very high depending on its color and translucency–up to $200 dollars per carat. Because of this, there are many fakes on the market like common clear or milky chalcedony that is died to look like gem silica, or even lessor expensive Chrysoprase (Chalcedony colored by Nickel impurities), can be sold under the Gem Silica name. So buyer beware!


Natural Botryoidal Gem Silica: photo credit to the Arkenstone
Gem Silica Cabochon: Inspiration Mine, Gila County, Arizona.
Chrysocolla
Formula: Cu2-xAlx(H2-xSi2O5)(OH4)•nH2O

-
A minerals that is always associated with secondary copper minerals.
-
A form of copper salt.
-
It is named for the greek words for gold-glue.
-
Comes in around 2.5 to 3.5 on the Mols hardness scale.
-
A member of the phyllosilicates group.
-
Chrysocolla is colored by the copper salts in the mineral.
-
It is blue to blue green in coloration, but is can be found in other colors.
Chrysocolla is a copper salt phyllosilicate (Silicate rings) with water in its structure. It might seem odd to have water in a mineral, but many minerals do have water as part of their chemistry. It is always found with other copper bearing minerals and around copper mines too.
It was names by the greek Theophrastus in 315 B.C. and comes from the Greek "chrysos", meaning "gold," and "kolla", meaning "glue," in allusion to the name of the material used to solder gold. André-Jean-François-Marie Brochant de Villiers revived the name in 1808.
It is a relatively soft and easily broken structure. It is typically found as glassy botryoidal or rounded masses or bubbly crusts, and as jackstraw mats of tiny acicular crystals or tufts of fibrous crystals. There are no known large crystals of Chrysocolla. The Chrysocolla "crystals" are all pseudomorphs.

Chrysocolla Formation: Powder-blue chrysocolla as stalactitic growths and as a thin carpet in vugs inside a boulder of nearly solid tyrolite from the San Simon Mine, Iquique Province, Chile (size: 14.1 x 8.0 x 7.8 cm)
Chrysocolla can often be covered and mixed in with Quartz crystals, Chalcedony and Calcite crystals making it very difficult to tell from real Gem Silica. The only way to know for sure is to have the specimen analyzed or looked at by an expert.
Sources: https://www.mindat.org/min-1040.html, https://www.ajsgem.com/articles/gem-silica-or-chrysocolla-chalcedony.html-0, https://geology.com/gemstones/gem-silica/, https://en.wikipedia.org/wiki/Chrysocolla, https://www.mindat.org/min-1040.html
Spessartine Garnet
Manganese Aluminum Silicate (Mn3Al2Si3O12)
Spessartine is member of the Garnet group, and is known for its aesthetic orange and reddish-orange colors. This form of Garnet was once much rarer, but new abundant finds in Tanzania, China, and Pakistan have really put Spessartine on the map, making it very well regarded. Spessartine forms a solid solution series with Almandine, and can be virtually indistinguishable from it in localities where both these Garnets occur together. Re-named in 1832 by François Sulpice Beudant after its type locality in the Spessart Mountains, Germany. Previously distinguished as a "manganesian" garnet by Henry Seybert in 1823 using mineral from Haddam, Connecticut, USA. Originally, this mineral, from Spessart Mountains, was called "granatförmiges Braunsteinerz" in 1797 by Martin Klaproth.
A new outstanding occurrence of bright orange Spessartine crystals in Tanzania was first brought to the market in 2008. The deposit is in Nani, Loliondo, Arusha Region, near the Serengeti National Park. Bright orange crystals once came from Marienfluss, Kunene Region, Namibia, but these high quality Spessartine forms are very hard to come across today. Another important African locality is the Jos Plateu, Nigeria. Malaya Garnet (a trade name for Garnet intermediary between Spessartine and Pyrope) is well-known from Mwakaijembe in the Umba River Valley, Tanzania.
Another recent outstanding discovery of Spessartine was in China, where it first discovered in the late 1990's in Tongbei and Yunling, Zhangzhou Prefecture. The Chinese Spessartine is often in dense aggregates of small gemmy crystals coating Smoky Quartz. The finest dark red Spessartine, usually associated with contrasting white Albite, comes from Pakistan at Shengus and the Shigar Valley, Skardu District; and in the Gilgit District. Spessartine of similar quality is also found in Darra-i-Pech, Nangarhar Province, Afghanistan.
Lustrous Spessartine, sometimes in complex crystals with deep etchings, comes from several of the gem pegmatite in Minas Gerais, Brazil, especially at Conselheiro Pena, São José da Safira, and Galiléia, all in the Doce valley. Especially noted is the Navegadora Mine in São José da Safira which produces heavily etched contorted crystals. Other worldwide Spessartine occurrences include Broken Hill, New South Wales, Australia; Val Codera, Sondrio, Italy; San Piero in Campo, Elba Island, Italy; and Iveland, Aust-Agder, Norway.
In the U.S., the most well-known occurrences of Spessartine are the Little Three Mine, Ramona, San Diego Co., California; the Pack Rat Mine, Jacumba, San Diego Co., California; Ruby Mountain, Nathrop, Chaffee Co., Colorado; East Grants Ridge, Cibola Co., New Mexico; and the Thomas Range, Juab Co., Utah.
Sources: Mindat.org, geology.com, minerals.com

Example of Spessartine Garnets from the Fujian Province, China.

Triangle Chemical Chart of the Pyralsprites garnet group that features Spessartine-Pyrope and Almandine garnet families.
.jpg)
Several natural uncut Spessartine garnets with cut gemstones made from the same group of crystals. Spessartine gemstones are most prized for their neon-orange to orange-red colors

Example of Spessartine Garnets on matrix as originally found after cleaning. Garnets are known for often form geometric crystals like decahedrons & isocahedron-like shapes.

Example of Spessartine Garnets on that have formed over Smokey Quartz in China.
What Makes Minerals Fluorescent?
Reprinted from Rock & Gem Digital Posting with additions. Original story by Bob Jones.

The short answer is that some minerals are self-activators. Others depend on some form of impurity that acts as an activator. Minerals that are fluorescent under ultraviolet light are beautiful and fun, however, the great majority of minerals do not respond with color under ultraviolet light. Estimates vary from 10 to 15 percent of the known 5,000 minerals may respond. Including the rare earth elements, there are over 30 different common elements and ions that can cause fluorescence.
Self-Activating Minerals
Self-activating minerals use their own electrons to absorb ultraviolet energy giving their electrons the energy to shift away from the atom’s nucleus to the next higher energy level, or orbital. The remaining light energy is out of balance and reemitted and can be seen as a visible color. Ultraviolet energy is not visible so what you see is the lower electromagnetic energy level resulting from the action of the activator.
What Makes Minerals Fluorescent - Activators
The great majority of activators are atoms of certain metal elements which become part of the mineral’s chemistry by taking the place of atoms in the host mineral. For example, sodium chloride halite normally lacks color but if trace manganese atoms are present, they make the halite glow a lovely red under short wave ultraviolet radiation. When the electrons in a responding mineral shift to a higher orbit they can’t stay there indefinitely. They are constantly shifting with blinding speed between their normal position and a higher orbital as the ultraviolet energy continues. Even though the electrons are shifting, the color we see is steady.
The Process
Fluorescent minerals contain certain atoms with electrons that are taken up to a higher excited energy levels by absorbing energy from the incoming ultraviolet light. These electrons instantly fall back to their original energy levels, giving off energy in form of visible emitted light. This takes place in a very small fraction of a second. We see only the resultant visible light emitted as long as the atoms are exposed to the ultraviolet light. Depending on the energy released when electron returning to their original level, minerals exhibit different fluorescence colors.
Known Activators
As we gain greater ability to pick apart a mineral, we are finding more activators at work and they are not all metal elements. Some are more complex ions. Activators like uranyl oxide are regular participants in many radioactive minerals even with the trace manganese. These common activators have joined with some odd elements you would not think could trigger a color such as lead (Pb) in hydrozincite and sulfur (S) in sodalite, a variety of hackmanite from Canada.
What Makes Minerals Fluorescent - Rare Earth Elements
Rare earth elements are common activators. You see these elements listed at the bottom of the Periodic Table because they share many of the same chemical, physical, and mineralogical properties and a similar electron configuration of two valence electrons in an outer orbital. Since rare earths often occur together in the same deposit, it is inevitable when an activator is present it can be any one of a suite of rare earths rather than just one element.
Two or more rare earth elements have been identified as causing fluorescence in some fluorite, strontianite, calcite, esperite, fluorapatite, powellite and scheelite. These last two are self-activating most often but can also respond to rare earths. The tungstate ion in scheelite is what responds to ultraviolet excitation, usually a brilliant blue under shortwave. In powellite, it is the manganese oxide ion that is the main activator causing a yellow response.
Why Activators Work
There is one other factor worth considering with activators. Why does an activator function only in certain minerals and not in all minerals? There are two reasons. The activator has to have a proper valence or number of electrons in its outer orbital, very often two. Its atoms also have to be close in size to the host atom that it replaces so it becomes part of the mineral’s chemistry and fits in the mineral’s lattice structure.
Manganese
Manganese is the most common activator. It is found in many of the minerals from the Franklin and Sterling Hill mining district in New Jersey causing the town of Franklin to be named the Fluorescent Mineral Capital of the World. Manganese is a transition metal element which means its outer orbital can hold a varying number of electrons, in this case, two-three or four. They can be shared and become the agent in chemical bonding.
Usually, it is manganese valence two that ends up as a trace metal serving as an activator. In the mineral calcite, for example, it has a valence of two and can replace some calcium atoms with a similar valence in the mineral’s lattice structure. Franklin-Sterling Hill calcite depends on manganese as its activator. The calcite can respond as a brilliant red. Studies have shown the optimum content of manganese activator in calcite at Franklin for a strong fluorescent response is about three percent. Too much of a good thing and the response is diminished, or not there at all.
The same valence two of manganese is also responsible for other fluorescent minerals from the Franklin mine. This is because these are zinc minerals and zinc has a valence of two.
Size Matters
How about the size of atoms? Zinc atoms are close enough in size to manganese that they can replace some zinc. Willemite easily accepts manganese atoms as an activator resulting in a bright fluorescent response but in this case green, not red.
What Makes Minerals Fluorescent - Other Activators
Other activators are not simple elements. Some minerals may contain a trace of organic material like natural oil and will fluoresce. I recall collecting fluorite that included organics in the quarry at Clay Center, Ohio. The pale brown transparent fluorite cubes had a creamy or slightly bluish color depending on the type of ultraviolet lamp used. Doubly terminated quartz crystals found in Herkimer, New York, may show fluorescence. “Herkimer Diamonds” developed in cavities created by organic stromatolites which existed millions of years ago. They died and left behind organic material which is picked up by the quartz as it forms. That’s what causes the fluorescence.
Recently Discovered Activators
We now know there is another group of activators not known decades ago consisting of two or three different elemental ions. Such things as carbon trioxide (CO3 ) in calcite or topaz may cause a response. Much more important in topaz is the activator titanium oxide ion (TiO6). California’s official gemstone is benitoite a titanium mineral. It fluoresces blue in short wave thanks to the titanium oxide ion (TiO3). Certainly, the most frequently seen ion as an activator is uranyl ion (UO2). It shows up in a host of radioactive minerals as well as other species.
(Back, Left to right) Fluorite, Scapolite, Willemite with Calcite and Franklinite (Front, Left to right) Willemite with Franklinite, Calcite, Opal var. Hyalite

Fluorescent Dugway Geode: Many Dugway geodes contain fluorescent minerals and produce a spectacular display under UV light! Specimen and photos by SpiritRock Shop.

This spectacular, 4-colored, fluorescent specimen is from the famous Franklin Mine, Franklin, Sussex County, New Jersey. Minerals are Clinohedrite, Hardystonite, Willemite, and Calcite.
Quenchers
While it seems that all radioactive minerals should fluoresce, they do not. Uraninite, the main uranium oxide mineral does not respond at all. A host of the popular radioactive minerals, like autunite, do fluoresce. But, the copper uranium mineral torbernite may not. This brings up the idea of quenchers, trace minerals that inhibit or prevent a fluorescent response.
Copper promotes good color in many minerals like azurite and malachite. If copper is present in non-copper species that might otherwise fluoresce, they will not. Copper quenches the fluorescence, but not always. Normally, adamite is just about colorless but a little copper gives it that rich lime green color. Mexican adamite will fluoresce a bright green color because of the uranyl ion. The fluorescent response varies from brilliant green to no response at all. It all depends on the copper-uranyl relationship controlling the effects of ultraviolet.
Another quencher is iron. But again we find a conundrum. Iron minerals don’t fluoresce. But the iron in trace amounts of a mineral can be an activator as in some feldspars like anorthoclase. It can also be an activator in petalite and pectolite, though they tend to react better with other activators. Iron ions are also responsible for many gemstone's colorations of blues and yellows in teh visible spectrum.
Unknown Activators
There are still a great number of minerals that fluoresce because of some unknown activator. A particular mineral species may or may not fluoresce depending on where it is found. This is what makes collecting fluorescent minerals so exciting. Coupled with the wide range of ultraviolet equipment, and the continuing discovery of more mineral species that fluoresce, the hobby will continue to grow.
Sources: https://www.earthsciences.hku.hk/shmuseum/earth_mat_1_2_6.php, https://geology.com/articles/fluorescent-minerals/, https://geology.com/articles/fluorescent-minerals/, https://www.naturesrainbows.com/post/clinohedrite-hardystonite-willemite-and-calcite-franklin-mine-franklin-new-jersey
For the March story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. Once again, these two minerals are named very similarly but are quite different.
So what's it gonna be?
Rosolite vs Rubellite!
While these two minerals share reddish names but they are from two completely different families within the silicates mega-group of minerals. Here is how they break down.
Rosolite
Formula Ca3Al2(SiO4)3 - Garnet Family
-
Usually found as Isometric and geometric crystals in small pockets.
-
A light pink to red variety of Grossular Garnet.
-
It is named for its pinkish-red color.
-
Comes in around 6.5 to 7 on the Mols hardness scale.
-
Sometimes called Raspberry Garnet for its appearance.
-
Garnets are a very diverse group of minerals that share a varied chemical structure with a silicate core.
Rubellite
Formula A(D3)G6(T6O18)(BO3)3X3Z - Tourmalines
-
Typically found in columnar prismatic crystals in the trigonal crystal habit
-
From the latin word 'rubellus' meaning 'reddish'.
-
Comes in at about 7 on the Mols hardness scale.
-
Large transparent red Rubellite crystals are rare and highly sought after by collectors.
-
The tourmaline family is a large and colorful family of minerals. Rubellite is the pink to red variety of Elbaite Tourmaline and is very rare.
-
Sometime mistaken for Ruby.

Rosolite Garnet Crystal from Lake Jaco area in Sierra de la Cruz, Coahuila, Mexico.

Rubellite in matrix from Jonas Mine, Conselheiro Pena, Doce valley, Minas Gerais, Southeast Region, Brazil
For Your Favorite Collector at the Holidays...
Six Rare and Collectable Gemstones & Minerals Ideas
There are probably thousands of rocks and minerals that we could call the rarest and most expensive that have come and gone, but what about the ones that are still somewhat readily collectible? If you want to surprise your favorite collector, here are some of the best rocks and minerals still available in a short list that should give you a great start:
Gem Silica
Chrysocolla is a beautiful blue mineral often mistaken for turquoise. Unfortunately, Chrysocolla in its purest form, is soft and brittle, making it unsuitable for use in jewelry. Occasionally, the same copper salts that give Chrysocolla its wonderful blue color, naturally stain normally colorless chalcedony quartz, giving it a wonderful translucent to transparent blue color. Natural Gem Silica is extremely rare and cabochons made from high-quality Gem Silica can cost more than $100 per carat. Other gemstones made from chalcedony include Chrysoprase and Carnelian. These gemstones, while beautiful, are not nearly so rare and are considerably less expensive. Due to the high demand and high price of quality Gem Silica, care should be taken when purchasing Gem Silica. There are many examples of lower quality non-transparent, Chrysocolla being sold today.


British ColumbianJade
There are two completely different types of Jade, Nephrite Jade, and Jadeite. British Columbia Green Jade is a type of Nephrite Jade. Jadeite is about the same hardness as quartz. Nephrite Jade is softer than Jadeite, however, it is much tougher (harder to break), making it ideal for carving and use in jewelry as well as non-traditional uses such as interior or exterior tiles. Described as the “toughest natural stone on earth”, Nephrite Jade is extremely hard to mine because traditional mining methods are virtually useless due to the toughness of the material, and using explosives proves damaging to the Jade.
Extreme high-pressure hydraulic splitters can be used if there are any existing fractures available in the jade however typically, the jade is removed by using huge circular diamond saws or diamond wire saws. Very short summer conditions also prevent British Columbia Jade from being extracted for a few months per year. Although the deposits of Nephrite Jade are quite extensive, the costs associated with extracting the material, along with the short summers and limited production, make it difficult to satisfy the demand for high-quality jade.
Russian Charoite
Russian Charoite is a beautiful lavender to deep purple gemstone with swirls of other colors such as green, black, and sometimes orange. The most notable characteristic of Russian Charoite besides the wonderful color is its chatoyancy or better known as the “cat’s-eye effect”. Russian Charoite was first discovered in the late 1940s although it did not become popular until recent years.
Over the last few years, the price for Russian Charoite has shot up dramatically. Those who were fortunate to invest in Charoite several years ago made a great investment indeed!


Meteor Rock
Rarer than gold, platinum, or diamonds, meteor rock, or meteorites, commonly known as fallen stars, can sell for many times the price of gold, sometimes selling for more than $300 per gram. These bits and pieces of space debris, survived their fiery descent through the earth’s atmosphere, landing on earth, just waiting to be found by treasure hunters. Each meteorite has its unique size and shape and is usually made up of stone or iron.
The most common meteorites are made of iron and nickel. If they are polished and acid-etched, they will display a wonderful geometric pattern that is highly sought after by collectors.
Victoria Stone
Seldom do man-made stones get classified as gemstones. Victoria Stone is one of those rare exceptions. Created by Dr. Imori beginning in the late 1960s, Victoria Stone was created using a mixture of natural minerals such as quartz, feldspar, calcite, and magnesite.
These natural minerals were melted and then were made to crystallize using his secret formula, creating a new rock that has wonderful patterns and chatoyancy (cat’s-eye effect). Unfortunately, Dr. Imori died before passing on his secret formula and the process has never been duplicated. As a result, Victoria Stone is quite rare today and commands a hefty price.


Lander Blue Turquiose
If we’re looking for the rarest and most valuable turquoise in the world, you would be looking for Lander Blue Turquoise. Lander Blue Turquoise was mined in Lander County, Nevada, and was first claimed in 1973. Less than 110 pounds of this fantastic bright blue spider-web turquoise was ever mined.
If you want to collect some of this rarest of turquoise, expect to pay over $200 per carat and that’s for the small cabochons.

Happy Hunting!
For the December story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. Once again, these two minerals are named very similarly but are quite different.
Zincite vs Zinkenite!
While these two minerals have a similar name, they are from two completely different families of minerals. Here is how they stack up to each other.
Zincinite
Formula Pb9Sb22S42 - Sulfosalts Family
-
Typically found in columnar crystals that strongly resemble stibnite. Small clusters of crystals can be needle-like formations.
-
Named after Johann Zinken and contains no Zinc.
-
Comes in at about 3.5 on the Mols hardness scale.
-
Its crystals are often an opaque metallic steely coloration.
Zincite is the mineral form of zinc oxide (ZnO). Its crystal form is rare in nature; a notable exception to this is at the Franklin and Sterling Hill Mines in New Jersey, an area also famed for its many fluorescent minerals. It has a hexagonal crystal structure and a color that depends on the presence of impurities. The zincite found at the Franklin Red coloration is mostly due to iron and manganese, and associated with willemite and franklinite.
Zincite crystals can be grown artificially, and synthetic zincite crystals are available as a by-product of zinc smelting. Synthetic crystals can be colorless or can range in color from dark red, orange, or yellow to light green.
Zinkenite is one of a few sulfide minerals that form fine acicular crystals that appear as hair-like fibers. The fibrous aggregates may be so thick as to cover a specimen with a mat of hair-like fibers or it may be sparsely disseminated between other minerals and may be confused with stray hairs or dark lint. Jamesonite, boulangerite, and millerite are other sulfides that form similar acicular crystals. These sulfides as well as zinkenite have been called "feather ores" because of this unusual habit. Zinkenite is a sulfosalt, a segment of sulfides where the antimony acts more like a metal than a non-metal and occupies a position where it is bonded to sulfurs. A variety of zinkenite from Tasmania contains small amounts of silver.

Orange-Red Zincite Crystals from a Polish Zinc Smelting Plant.

Zinkenite Crystal from the San Jose Mine in Bolivia
Red Beryl
A Gem ‘Rarer Than Diamond and More Valuable Than Gold’



"What Gemstone is found in Utah that is rarer than diamond and more valuable than gold?” That was the compelling headline penned in 2002 by the Utah Geological Survey to introduce its readers to red beryl, a little-known gemstone found primarily in the state’s Wah Wah Mountains.
Discovered in 1904 by Maynard Bixby, this raspberry-red gem had the bookkeeper-turned-miner scratching his head. He had a hunch that the stunning crystals represented a variety of beryl, but the red color didn’t correlate with any beryl known to exist at the time.
Today, the best-known varieties of beryl include emerald (green), aquamarine (blue), morganite (pink), and heliodor (yellow). One year after Bixby’s discovery, W.F. Hillebrand, a geochemist from the National College in Washington, D.C., confirmed that Bixby’s find was a new type of beryl. In 1912, Dr. A. Eppler named the fiery gem “bixbite” in his honor.
The name beryl comes from the Greek word “Beryllos” which means sparkling or brilliant. The well-known varieties of beryl include emerald, aquamarine, and morganite. With Mohs hardness of 7.5-8.0, they are not as hard as topaz, rubies, sapphires or diamonds, but they are all suitably hard for jewelry applications.
Over time, bixbite assumed several names, including “red emerald” and the more proper “red beryl.” The name bixbite fell out of favor because it was often confused with bixbyite, a black manganese iron oxide also discovered by Bixby, in 1897.
Even though more than 100 years have passed since Bixby first encountered the curious red variety of beryl, the mineral has been unearthed in just a few locations — Utah’s Thomas Range, Utah’s Wah Wah Mountains, and New Mexico’s Black Range. The extremely rare variety of the mineral which gets its red color from trace amounts of manganese, red beryl has only been discovered in Utah, New Mexico, and Mexico. Furthermore, the Ruby Violet mine in the Wah Wah Mountains of Utah closed in 2001, is the only source in the world that has provided crystals suitable for cutting. The Utah Geological Survey estimated that one crystal of red beryl is found for every 150,000 gem-quality diamonds. In 2006 the Jewelers Association designated red beryl as the world’s rarest colored gemstone.
Of the three, only the Wah Wah Mountains have produced gem-grade crystals that are large enough to be faceted. The gems are primarily sourced at the Ruby-Violet Claim in Beaver County, Utah. The best specimens of red beryl display a raspberry-pink to slightly purplish-red color.
Writing for the Utah Geological Survey, Carl Ege noted that red beryl was worth 1,000 times more than gold and was so rare that one red beryl crystal is found for every 150,000 diamonds. Because red beryl is rarely found in large sizes, the Gemmological Association of Great Britain estimated that a 2-carat beryl has the same rarity as a 40-carat diamond.
The British gem association reported that the largest known faceted red beryl weighs in at 8 carats.
Gemsociety.org wrote that most fine red beryl crystal specimens are “zealously guarded by mineral collectors and never faceted.” The one shown, above, is part of the Smithsonian’s National Gem Collection in Washington, DC.
Sources: https://www.igi.org/gemblog/whats-150000-times-more-rare-than-diamond/, https://instoremag.com/red-beryl-a-gem-rarer-than-diamond-and-more-valuable-than-gold/
For the September story, we're featuring two minerals in a head-to-head shoot-out of chemistry and qualities. Once again, these two minerals are named very similarly, but are quite different.
So what's its gonna be?
Rhodonite vs Rhodochrosite!
While these two minerals are related in many ways, they are from two completely different families of minerals. Here is how they breakdown.
Rhodonite
Formula CaMn3Mn[Si5O15] - Inosilicates Family
-
Rarely found as tabular red crystals, usually in clusters with the crystals growing parallel to one another, or nearly so. Also as pink masses with other metallic minerals.
-
Named after the greek word for rose.
-
Comes in around 6 on the Mols hardness scale.
Rhodochrosite
Formula MnCO3 - Calcite Family
-
Typically found in rhombic (square-ish) crystals or as stalactites, kind of like calcite often forms. Polished and tumbled stones can resemble rose quartz.
-
It is also named from the greek for rose coloring.
-
Comes in at about 3.5 on the Mols hardness scale.
-
Large translucent red Rhodochrosite crystals are rare and highly sought after by collectors.
Rhodonite is a pink manganese silicate mineral of variable composition that often contains significant amounts of iron, magnesium, and calcium. It has a generalized chemical composition of (Mn,Fe,Mg,Ca)SiO3. Rhodonite is often associated with black manganese oxides which may occur as dendrites, fracture-fillings, or matrix within the specimen. Other names for rhodonite include "manganese spar" and "manganolite." Rhodonite is an uncommon mineral. It is found in a few small deposits across the world.
Rhodochrosite is a manganese carbonate mineral that ranges in color from light pink to bright red. It is found in a small number of locations worldwide where other manganese minerals are usually present. Rhodochrosite is sometimes used as an ore of manganese but is rarely found in economic quantities. Specimens with a wonderful pink color are used to produce highly desirable gemstones. Rhodochrosite is rarely found as well-formed crystals, so crystals can be extremely valuable as mineral specimens.

Pink & White - Rhodochrosite!

Red Rhodochrosite Dogtooth Crystal

Rhodochrosite stalactite slab cross-section

Pink & Black - Rhodonite!

Rhodonite Cabochons
This month's article is going to be a bit different. Instead of featuring one mineral or fossil, were going to have a skirmish. A head-to-head contest between two minerals who happen to have names that are soooo similar that even mineral collectors will pause and have to think about what it is that we're talking about. We plan to do this a few more times in the hopes of making it fun and provide some learning about the mineral world as well.
So what's it gonna be?
Baryte vs Beryl!
While these two minerals are related in some ways, they are from two completely different families of minerals, and to add to the confusion, one often gets spelled differently too. Now for the breakdown.
Baryte
Formula BaSO4 - Sulfate Family
-
Typically found as thick to thin tabular crystals, usually in clusters with the crystals growing parallel to one another, or nearly so. Also as bladed, white masses or flowery like clusters of crystals.
-
Named for its heaviness as a non-metallic mineral.
-
Comes in at a 3 on the Mols hardness scale.
Baryte is often written as Barite and it's fairly soft for a mineral. Too soft to make gemstones out of, but it can be very pretty forming transparent blue, yellow, and clear crystals. The mineral baryte is mined as a source of Barium and is used in many industrial products and processes. Many mineral collectors will have a few pieces in their collection for their interesting shape, colors and it's not too expensive for some really interesting pieces.

Blue Baryte

Yellow Baryte
Beryl
Formula Be3Al2(Si6O18) - Cyclosilicates Family
-
Typically found in hexagonal columnar crystals.
-
Its name is so old that we guess it came from the greek "beryllos" which referred to a number of blue-green stones in antiquity.
-
Comes in at a 7.5 to 8 on the Mols hardness scale.
-
Beryls are known by different names based on their color, like green for emerald.
Beryl is known for its stunning rainbow of colors. It is prized as a gemstone in jewelry. Prized beryls come in red (Bixbite), green (Emerald), light blue (Aquamarine), yellow (Heliodor), purple-blue (Maxixe), pink (Morganite), and colorless (Goshenite). Many people favor Beryls over diamonds and rubies as their favorite gemstone. Large and tall beryl crystals of clean, bright colors are highly prized by collectors and gemologists.

The Colors of Beryl
The Biggest Bug ever!
The Prehistoric Dragonfly with a Two-foot Wingspan

Image Credit: Fossil of Meganeuridae
The largest known insect of all time was a predator resembling a dragonfly but was only distantly related to them. Its name is Meganeuropsis permiana, and it ruled the skies before pterosaurs, birds, and bats had even evolved.
Most popular textbooks make mention of “giant dragonflies” that lived during the days before the dinosaurs. This is only partly true, for real dragonflies had still not evolved back then. Rather than being true dragonflies, they were the more primitive ‘griffin flies’ or Meganisopterans. Their fossil record is quite short. They lasted from the Late Carboniferous to the Late Permian, roughly 317 to 247 million years ago.
Meganisoptera is an extinct family of insects, all large and predatory and superficially like today’s odonatans, the dragonflies, and damselflies. And the very largest of these was Meganeuropsis. It is known from two species, with the type species being the immense M.permiana. Meganeuropsis permiana, as its name suggests is from the Early Permian timeframe.
The fossils of Meganeura were first discovered in France in the year 1880. Then, in 1885, the fossil was described and assigned its name by Charles Brongniart who was a French Paleontologist. Later in 1979, another fine fossil specimen was discovered at Bolsover in Derbyshire.
There has been some controversy as to how insects of the Carboniferous period were able to grow so large.
Some leading Ideas are that oxygen levels and atmospheric density were different during the early Permian.
The way oxygen is diffused through the insect's body via its tracheal breathing system puts an upper limit on body size, which prehistoric insects seem to have well exceeded. It was originally proposed that Meganeura was able to fly only because the atmosphere at that time contained about 15% more oxygen than the present 20%.
Another explanation for the large size of Meganeurids is comparing it to living predators is warranted. It was suggested in 2004 that the lack of aerial vertebrate predators allowed pterygote insects to evolve to maximum sizes during the Carboniferous and Permian periods, perhaps accelerated by an evolutionary "arms race" for an increase in body size between plant-feeding Palaeodictyoptera and Meganisoptera as their predators.
Another theory suggests that insects that developed in water before becoming terrestrial as adults grew bigger as a way to protect themselves against the high levels of oxygen. They grew in size simply because the ecosystem allowed them to, and the increased levels of oxygen have been shown to help today's insects grow larger when kept in an oxygen-rich atmosphere.
Interesting Facts
-
Though always associated with the modern-day dragonflies due to their appearance, considering the various structural and other characteristic differences between them, these insects were often classified as griffin flies.
-
It was one of the largest known insects that ever lived, with a reconstructed wing length of 330 millimeters (13 in), an estimated wingspan of up to 710 millimeters (28 in), and a body length from head to tail of almost 430 millimeters (17 in).
-
The term 'Meganeura' means large-veined, and these insects had similar vein patterns in their wings. However, the vein patterns found in the wings of dragonflies usually vary.
-
It is believed that their hunting and preying methods were quite similar to those of modern-day dragonflies. However, it may have attacked many more creatures because of its larger size.
-
Their wings had a network of veins. Moreover, they were heavily veined and had cross braces for strength unlike those of the present-day dragonflies that have delicate wings.
-
They believe that it was impossible for the massive bodies of these insects to survive in the present-day atmospheric conditions and that this may have led to their extinction. (The oxygen content in today's atmosphere is up to 21% and back in the Carboniferous period, it was up to 35%.)
-
The breathing mechanism of these insects allowed the passage of air through a system of tracheal tubes, transporting the oxygen directly to the internal tissues.

Size Comparison

Today's Dragon Fly Larvae

Fossil of a Dragonfly Larvae
A New Russian Mineral Discovery that's More than a Pretty Face
Petrovite
A research team led by crystallographer (crystal specialist) Stanislav Filatov at St. Petersburg University found a colorful new entry into the world of minerals: petrovite. Petrovite is beautiful to look at, but it could also help inspire advancements in next-generation batteries.
The research team that found petrovite was headed by crystallography professor Stanislav Filatov, who studied the minerals of Kamchatka for over 40 years. The area offers amazing mineralogical diversity, with dozens of new minerals found there in recent years, according to the university's press release. Specifically, Filatov focused his attention on scoria (or cinder) cone volcanos and lava flows formed after the eruptions of the Tolbachik Volcano.
The bright blue mineral comes from a wild place: a volcanic landscape formed by major eruptions in the 1970s and the 2010s in the Kamchatka Peninsula of Russia. "This territory is unique in its mineralogical diversity. In recent years, researchers have discovered dozens of new minerals here, many of which are one-of-a-kind in the world," the university said in a statement on Tuesday.
The mineral is named for another St. Petersburg University crystallographer, Tomas Petrov. The team published a study on petrovite in the journal Mineralogical Magazine earlier this year.
Petrovite is particularly interesting because it's a rarity in its composition and structure. Petrovite is a blue-green mineral, with the chemical formula of Na10CaCu2(SO4)8. "The mineral consists of oxygen atoms, sodium sulphur and copper, which form a porous framework," the university states. "The voids are connected to each other by channels through which relatively small sodium atoms can move."
The scientists think its structure of voids connected by channels, which can pass through small sodium atoms, holds potential for ionic conductivity. The mineral may be adaptable as cathode material in sodium-ion batteries. Due to the abundance of salt, sodium-ion batteries could be a very inexpensive alternative to lithium-ion batteries you can commonly find in many devices today.
Besides researchers from St. Petersburg University, other Russian scientists involved came from the Institute of Volcanology and Seismology of the Far Eastern Branch of the Russian Academy of Sciences, and the Grebenshchikov Institute of Silicate Chemistry.
Petrovite was born in a fiery place in the wild, but Filatov said researchers could look into synthesizing a compound with its same structure in a lab for use in battery development. That would be quite a journey from a volcano to powering gadgets in people's homes.
Sources: https://www.cnet.com/news/scientists-discover-beautiful-blue-new-mineral-petrovite/, https://en.wikipedia.org/wiki/Petrovite, https://bigthink.com/surprising-science/newly-discovered-mineral-petrovite-could-revolutionize-batteries?rebelltitem=3#rebelltitem3, Cambridge University Press.


The Chemical Structure of Petrovite with copper centers surrounded by seven oxygen atoms shared in silicate tetrahedrons
Petrovite found in the Kamchatka Peninsula of Russia with a color that gives clues to its copper-centered chemistry.
Finding amazing Fossils
All of us have the potential to find a once in a lifetime find, including a 4-year-old girl from England.

© Amgueddfa Cymru - National Museum of Wales
The 10-cm long footprint was discovered by Lily Wilder near Bendricks Bay in south Wales, on January 30, 2021.
Paleontologists stunned by a perfectly preserved dinosaur footprint discovered by a 4-year-old girl
by Sophia Ankel (sankel@businessinsider.com)
Lily Wilder made the discovery on January 23 while walking along a beach in South Wales with her father and dog. The family was on their way to the supermarket when Wilder saw the footprint imprinted on a rock.
"It was on a low rock, shoulder height for Lily, and she just spotted it and said, 'look, Daddy,'" her mother, Sally Wilder, told NBC News. "She is really excited but doesn't quite grasp how amazing it is."
At first, the family thought the print, which is just over 10 cm (4 inches) long, was scratched out on the rock by an artist. But mother Sally was aware that similar footprints had been found along that piece of the coast before, so she posted about their discovery on social media.
"I found this fossil identification page on Facebook and I posted it on there and people went a bit crazy," she told Wales Online. Shortly after, The National Museum of Wales was in touch with the Wilder family, and officials have since retrieved the print and put it in the museum. The family says their daughter's interest in dinosaurs has been ignited since the discovery and that she's been playing with a collection of dino toys and models.
Experts believe the footprint was most likely left by a dinosaur that stood about 75 centimeters (29.5 inches) tall and 2.5 meters (about 8 feet) long and walked on its two hind feet. It is impossible to identify exactly what type of dinosaur left it, although experts typically classify the print as a Grallator.
Welsh scientists are calling the girl's discovery "the finest impression of a 215 million-year-old dinosaur print found in Britain in a decade," according to Wales Online. "It's so perfect and absolutely pristine. It's a wonderful piece," said Karl-James Langford from Archeology Cyrmu, according to Wales Online. "I would say it's internationally important and that is why the museum took it straight away. This is how important it is. I would say it's the best dinosaur footprint found in the UK in the past 10 years," he added.
The National Museum in Cardiff, which is currently closed due to the COVID-19 pandemic, said that Lily and her classmates would be invited to the exhibition once it reopens. "What's amazing is, if her name goes down as the finder in the museum, it could be her grandchildren going to visit that in the museum one day, and for years and years and generations to come, which is quite amazing," mother Sally told Wales Online.
Go out and find your amazing find of a lifetime!
Proustite



From Minerals.net
Proustite is an interesting mineral that contains silver in its chemical structure. It is one of the few silver-bearing minerals that can exhibit transparency. Proustite is usually transparent, with deep-red crystals, but may also be a darker, more metallic-looking form. However, even darker, more metallic Proustite will be visibly red and transparent when backlit.
Proustite is light sensitive. Prolonged exposure to bright light will darken its transparency and cause it to become darker. Exposure also may cause a dark, dull film to form on crystal faces; this film can be removed by brushing a specimen with soap and water.
Proustite is very similar to Pyrargyrite, and forms a series with it. Proustite is the arsenic-rich member, and Pyrargyrite is the antimony-rich member. It is often not possible to visually distinguish these two minerals from each other, though Proustite is usually lighter in color. Most good material in collections today are from closed, historical localities. Several classic European localities have produced highly desirable Proustite specimens. Relatively large crystals have come from the Erzgebirge in Germany at Freiberg, Schlema, and the Schneeberg Districts. Across the border in the Czech Republic, some of the earliest sources Proustite have come from Jáchymov, Krušné Hory Mts, Bohemia. Small Proustite crystals, often associated with Quartz, were once found in the Ste Marie-aux-Mines, Haut-Rhin, Alsace, France.
A more recent producer of good Proustite crystals is Morocco, at the Imiter and Bou Azzer mines. In South America, some of the best examples of this mineral have come from Chañarcillo, Copiapo Province, Chile; and the Uchucchacua Mine, Oyon, Lima Department, Peru. In Canada, good crystal clusters and crusts of Proustites have come from the Cobalt region, Timiskaming District, Ontario
It forms prismatic crystals, often complex in form. Crystals are often elongated scalenohedrons with complex terminations. Also in blocky groups of stubby crystals, interpenetrating crystals, grainy, encrusting, botryoidal, globular, and massive. May also form in intergrowths of three crystals, forming a trilling. Crystals are usually striated horizontally on an angle and may have complex growths and angles.



Named by François S. Beudant in 1832 in honor of Joseph-Louis Proust (26 September 1754, Angers, France – 5 July 1826, Angers, France), chemist and actor, for Proust's work on the red silver minerals (proustite-pyrargyrite series). He is most famous for discovering the law of definite proportion, stating that chemical compounds always combine in constant proportions.
It has several other names that it is often called like Ruby Silver, Red Silver, Tears of Jesus, Blood of Christ, and Red Silver Ore. Proustite is a sulfosalt mineral consisting of silver sulfarsenide, Ag3AsS3.
Sources: https://www.minerals.net/mineral/proustite.aspx, https://en.wikipedia.org/wiki/Proustite,
How Squishy Animals Evolved Strong Shells and Bones
From Futury, Science Magazine and the NCBI

Credit: Pexels
The animal kingdom abounds with creatures that grow hard shells, carapaces, and skeletons. But complex life was pretty squishy at the beginning. A new study clarifies how and when things changed.
Animals with skeletons did not exist before about 550 million years ago. Then, scientists have proposed, atmospheric oxygen levels rose and the chemistry of the oceans changed in such a way that animals could harness the minerals required to build hard structural parts. A new analysis of ancient rock layers in Siberia provides support for this idea, showing that the oceans became rich in skeletal building blocks around the same time the first fossils of animals with skeletons start to appear.
Researchers discovered that when carbonate skeletons were first evolving more than 500 million years ago, diverse groups of animals all converged on a similar, counterintuitive process for biomineralization. Today, many unrelated animals build their skeletons or shells out of calcium carbonate—including echinoderms, mollusks, and corals. Instead of building crystals ion-by-ion from the surrounding seawater, these animals use amorphous, or non-crystalline, nanoparticles as their building blocks of choice.
“In fact, crystallization by particle attachment actually seems to be the prevailing method of biomineralization as far as we can tell,” says Susannah Porter, a professor of earth science at the University of California, Santa Barbara.
Rather than building their skeletons at a molecular level, these animals first form nanoparticles of amorphous calcium carbonate. They then store these particles in vesicles that they can use to transport them to the site of crystallization. This method of crystallization was first documented more than 20 years ago in the teeth of sea urchins. Since then, scientists have noticed the process throughout the animal kingdom and involving different
minerals. What’s more, the different groups of animals seem to have independently settled on this method of biomineralization, so it must have something going for it. Given its ubiquity, Porter and her collaborators wanted to determine how far back they could find evidence of this process. Their findings appear in PNAS.
“We obviously can’t watch these Cambrian and Ediacaran organisms make their skeletons, so we need to have a proxy,” Porter says. First author Pupa Gilbert, of the University of Wisconsin-Madison, had previously found that crystallization by particle attachment leaves an irregular particulate texture in the shells and skeletons when they’re viewed under a scanning electron microscope. The team saw this same tell-tale pattern upon imaging fossils more than 500 million years old. In fact, this signature preserved even in the material that had subsequently converted into another mineral.
“It’s spectacular,” Porter says, “the fact that we can see this detail at the sub-micrometer level.” Among the ancient material, Porter and her collaborators examined were fossils of Cloudina, a genus that includes some of the earliest animals that formed a mineralized skeleton. The genus was named after Preston Cloud, a late professor of biogeology and preeminent researcher in the study of early life. The team saw the same irregular nanoparticulate texture in Cloudina fossils as in other animals that form crystals by particle attachment. “This shows that even when animals were first evolving mineralized skeletons, and were maybe not so good at biomineralizing, they were already choosing this mechanism,” Porter says.
The findings suggest that, even early on, there was a selection for this particular mechanism across different
lineages. “When you see something that is selected for over and over again, it suggests that it is the most
advantageous one,” Porter says.
Although it’s counterintuitive that animals would use amorphous material to create the crystals that ultimately form their skeletons or shells, Porter says that this mechanism seems to permit greater control over mineralization than simply building ion by ion, as the traditional models suggested. For one, these particles are incredibly stable when confined in vesicles: The material doesn’t immediately crystallize but remains amorphous. This allows the animal to keep ingredients around and available yet maintain flexibility regarding when and where the mineralized skeleton forms.
Additionally, compounds like calcium carbonate can take different structures—thereby forming different minerals—depending on environmental conditions. By storing the molecules in an amorphous state, the animal can better control what form, or polymorph, they become, Porter explains.
“It’s like having some frozen cookie dough around that you’re later going to bake into cookies,” she says. Porter is interested in the large-scale patterns of when lineages first evolved skeletons and how environmental and ecological conditions of the time affected those skeletons. She suspects that the earliest biomineralizers, like Cloudina, didn’t have particularly strong control over the process of building their skeletons. “But by the time you get to the Cambrian, the carbonate mineralizers have shells that are complex and organized,” she says. “They have much greater control over their skeletons.”

Specimens of Cloudina, which, known from sites around the world, is one of the earliest skeletal animals
Shuhai Xiao
VIRGINIA TECH
To understand the link between rising oxygen levels and the evolution of skeletons, Rachel Wood, a geobiologist at the University of Edinburgh, and her team studied ancient rock layers found deep in Siberia’s wilderness near the Yudoma River. The rocks, formed from layers of sediment deposited in ancient oceans, contain not only fossils but also sedimentary clues to how the oceans’ chemistry shifted during the time when skeletons are thought to have arisen. Wood spotted a series of ocean chemistry shifts during the Ediacaran and Cambrian periods, which together stretch from about 635 to 485 million years ago. Until roughly 545 million years ago, the rocks are rich in the mineral dolomite, which is believed to have formed in the oceans when oxygen levels were low, Hood says. After that, as levels of atmospheric oxygen increased, limestone rock predominates.
The limestone at the field site contains the minerals aragonite and calcite, which animals need to build skeletons; aragonite and calcite crystals form much faster and with less energy than dolomite, allowing animals to harness them for skeleton building in a way they can’t with dolomite.
This skeletal revolution is reflected in the rocks themselves. In the dolomite-rich layers, the fossils of soft-bodied organisms predominate—like Aspidella, a soft, frond-shaped creature that anchored itself to the seafloor. Then, in the limestone, one of the first known skeletonized animals appears—Cloudina, a millimeter-scale creature made of a calcified shell that looks like a stack of ice cream cones. From such beginnings, skeletonized animals would go on to evolve into such familiar forms as fish, shellfish, dinosaurs, and, eventually, humans.
As much as exoskeleton added speed to the evolution of animal life in general and created opportunities for animals to expand their activity radius by using calcified extremities and protection shields, it also imposed limitations, associated mostly with limited body size and lack of surface sensory organs. In addition, rigid shells and shields did not allow much movement and locomotion; therefore, the next major change in the evolution of skeleton—dislocation of mineralized skeleton from the outside to the inside of animal bodies, proved to be a major adaptive advantage. Especially in animal lineages that later gave rise to vertebrates, the appearance of endoskeleton enabled the expansion of activity radius and habitation of entirely new environments (Bennet 1991). In addition, those developments encouraged the development of a strong muscular system and added further adaptive values such as greater overall mobility and the appearance of a regenerative and environment-sensitive outer dermis (Ruben and Battalia 1979, Ruben and Bennett 1980).
Another major advantage of the architecture of mineralized skeleton was the development of an attribute of bone that decisively set vertebrates apart from virtually all other multicellular eukaryotes. The hard mineral fraction consisting mainly of calcium carbonate, which had been used over millions of years to build all forms of marine exoskeletons, was replaced by calcium phosphate, mostly in the form of calcium hydroxyapatite (Ruben and Bennett 1981, Ruben and Battalia 1992, Omelon et al. 2009). But why did vertebrates choose an entirely new mineralization strategy, and what special properties of calcium hydroxyapatite led to its integration into early vertebral skeletons?
A possible advantage of the novel chemical composition of vertebrate skeletons might be that calcium hydroxyapatite building blocks provide greater chemical stability. This may have been important, especially in the acidic environments created after bursts or periods of intense physical activity—conditions that are typical of most vertebrate species (Ruben and Battalia 1979, Ruben and Bennett 1981). Hydroxyapatite builds a more stable mineral component of the skeleton than can be achieved with a calcitic material, which is particularly important at pH ranges that are associated with the intense activity and a high-energy consuming lifestyle typical of vertebrates.
So which came first: The Tooth or the Shield?
In the earliest skeletal structures in vertebrate fossils, the tooth-like structures came before animals eventually developed boney dental-like structures for the protection of the skin. Early teeth and the forerunners of bony skin plates appear to be the product of the same genetic machinery, regulating epithelial/mesenchyme interactions and able to produce similar structures at different locations.
The Nine Scariest Rocks & Minerals
Inspired by Forbes Magazine (Feb 14, 2016)
Lead found in Flint, Michigan water is an unfortunate example of negligence leading to the mass exposure of elemental lead found naturally or man-made. Much in the same way humans communicate warnings of deadly plants and animals, it's important to communicate the risks and exposure of deadly rocks and minerals.
It's easy to forget how lethal our natural world can be, where an encounter with the wrong rock or mineral could lead to injury or death. Often times toxic minerals are associated with materials we use every day for construction, computers, and cosmetics. With a keen eye and an understanding of toxicity, you can help to identify deadly minerals in your surrounding.
The Deadliest Rocks & Minerals -
Chalcanthite - CuSO4·5H2O
The brilliant blue Chalcanthite is hydrated water-soluble copper sulfate. The mineral is used to ore copper, however, it's necessary to keep the environment dry as the mineral can easily dissolve and recrystallize in a wet environment. The water solubility of this mineral can easily lead to copper poisoning of an environment and is toxic to humans.

Stibnite - Sb2S3
Stibnite is a toxic antimony sulfide mineral with an orthorhombic crystal lattice and a source of metalloid antimony. Stibnite paste has been used for thousands of years for cosmetics to darken eyebrows and lashes. The mineral was also used to make eating utensils, causing poisoning from antimony ingestion.

Asbestos - Mg3Si2O5(OH)4
You have likely heard of the mineral asbestos and associate it with lung cancer. This silicate mineral grows thin fibers crystals that can easily break off and form dust particles. Despite its usefulness in insulation, fire resistance, and sound absorption, the mineral dust is deadly if inhaled. The fibers can cause lung cancer, mesothelioma, and asbestosis.

Arsenopyrite - FeAsS
Arsenopyrite is an iron arsenic sulfide with a brilliant steel metallic color often found in hydrothermal vents and pegmatites. The arsenic leads to a number of environmental and human dangers and can sometimes be associated with gold deposits. Oxidation of arsenopyrite can lead to soluble arsenic in water and subsequent arsenic poisoning of the groundwater.

Cinnabar - HgS
Cinnabar is a deep red mercury sulfide mineral that provides much of the world's elemental mercury. Despite the brilliant red color and history of use in trading and as a coloring agent, Cinnabar is deadly. Mercury is toxic to humans and was a source of death from many mines around the world. Ironically, long ago some cultures considered Mercury to be a longevity agent and consumed it leading to death.

Realgar - As4S4
Realgar is an arsenic sulfide mineral, also known as "ruby sulfur" or "ruby of arsenic". It is very pretty and another bright red "gemmy" looking mineral. It was used by firework manufacturers to create the color white in fireworks prior to the availability of powdered metals such as titanium. It is still used in combination with potassium chlorate to make a contact explosive known as "red explosive" for some types of torpedoes and other novelty exploding fireworks branded as 'cracker balls'. Realgar is toxic. It is sometimes used to kill weeds, and rats.

Hutchinsonite - (Tl,Pb)2As5S9
Hutchinsonite is a form of arsenic sulfide with thallium and lead that can be found in hydrothermal vents. Thallium salts are nearly tasteless and highly toxic and have been used in rat poison and insecticides. The thallium inclusion in this arsenic sulfide combines two extremely dangerous and deadly minerals. Exposure to this mineral can potentially lead to death.

Torbernite - Cu(UO2)2(PO4)2 · 8 - 12 H2O
Torbernite is a dangerous mineral composed of hydrated green copper, phosphate, and uranyl. The mineral is often found in granites that contain uranium and is dangerous due to its radioactive nature. The mineral releases radon naturally and can cause lung cancer if exposure is long enough.

Uraninite - UO2
Uraninite is a uranium oxide mineral and the most important ore of uranium. Uraninite is highly radioactive and should be handled and stored with care. “Pitchblende” is an archaic name that was used for uraninite and other black materials with a very high specific gravity into the late 1800s and early 1900s.

Sources: From Forbes Magazine and Senior Contributor Trevor Nace found at https://www.forbes.com/sites/trevornace/2016/02/14/9-deadliest-rocks-and-minerals-on-earth/?sh=1a5e6a43659b and Wikipedia images and realgar article. Geology.com was used for Uraninite.
Grow your own Purple Aluminate Crystals at Home


Hi MMS Fans!
In this above videos, we'll demonstrate to you a generally accepted method to grow a wonderful purple single beautiful crystal. For this, we'll require the accompanying substances – potassium and chrome alum. To start, how about we make a blend of alum that can be grown into beautiful octogram crystals.
To do this, take a glass and weight 100 grams of aluminum potassium sulfate and 12 grams of chromium potassium sulfate in it. Including the chromium aluminate will make the solution turn a deep purple color. At that point, pour 400 ml of extremely high temp (over 150 degrees F but not boiling) pure water into the glass and blend until the point when all the alum is dissolved into the water. Very hard water can ruin the process.
Carefully stir the solution until it's all dissolved in the water, leave this glass for a couple of days to give the gems a chance to form at the bottom of the glass container. After a day, carefully empty the alum arrangement into another container and set it aside, do not discard it. You should find a number of small lovely purple crystals that formed at the bottom of the glass. Pick open the mass of the precious stones and place them in a clean glass bowl. Look over the crystals that you pulled out and select the best looking or most interesting crystals. The selected crystals will be the seeds from which a larger crystal will be grown later on.
Tie one of the best seed crystals on a thin fishing line and hang it on a pencil or a stick so it's tied in the middle and hangs down. The best method is a slip knot, which you can look up how to do. Just make sure that it is firmly tied in place and remove any excess fishing line that hangs off the knot. You will be placing this in the solution that you set aside earlier. The solution is a saturated solution if you follow the measurements above. So, as the water evaporates out of the container or glass that you put it in, the excess aluminates will attach themselves to the seed crystal that is floating on the fishing line that you tied the seed crystal in the slip knot. If you want, you can do this with a few of the better crystals you pulled out at the beginning in case your experiment goes wrong or you need to start over. Only do one crystal at a time to get the best results.
Also, remember that you don't want any impurities to get into the solution, so it needs to be someplace that's clean and avoids temperature extremes for the best results. Even dust or flying bugs can spoil the process. You will need to keep an eye on the glass container to make sure that no crystals are forming on the bottom or on the string and remove them. Reheat your solution as shown in the videos and filter it into a new glass vial. Always remember to wear protective equipment and do this only as needed. The more you disturb the process, the more likely that something will go wrong, but if you heed the videos you should get good results.
Over a couple of months, the crystal grows larger and you can decide when its large enough that you want to stop its development. When your satisfied, remove it from the solution and dry its surface with a napkin or paper towel. Once it's completely dry, you can seal it with a couple of layers clear fingernail polish or clear enamel paint. Seal the surface in steps so that it can dry properly between coats. Once the paint is dry, it's safe to handle it and show it off!
And always remember that when you handling chemicals, wear protective equipment and be safe!
Fluorescence in Minerals
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light is in the visible region, which gives the fluorescent substance a distinct color that can be seen only when exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after.
Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labeling, dyes, biological detectors, and radiation detection. Fluorescence also occurs frequently in nature in some minerals and in various biological forms in many branches of the animal kingdom (from wikipedia).
Examples of Fluorescent Minerals and some of their properties:
Calcite Example

Calcite has been known to fluoresce red, blue, white, pink, green, and orange. Some minerals are known to exhibit multiple colors of fluorescence in a single specimen. ... (Left) Calcite under normal lighting, (Right) Calcite under Short Wave UV Raditons that causes it to emit light in the visible red spectrum. This particular Calcite is from South Africa.
Yooperlite Example
Recently discovered by Erik Rintamaki, Yooperlite® rocks are actually Syenite rocks that are rich in fluorescent Sodalite. Yooperlites® can be searched for on Michigan beached using a good UV spectrum LED flashlight and are usually found In the Upper Peninsula of Michigan, anywhere from Whitefish Point to Grand Marais.
Fluorite...The original Fluorescent Mineral

One of the first people to observe fluorescence in minerals was George Gabriel Stokes in 1852. He noted the ability of fluorite to produce a blue glow when illuminated with invisible light "beyond the violet end of the spectrum." He called this phenomenon "fluorescence" after the mineral fluorite. The name has gained wide acceptance in mineralogy, gemology, biology, optics, commercial lighting, and many other fields. Many specimens of fluorite have a strong enough fluorescence that the observer can take them outside, hold them in sunlight, then move them into the shade and see a color change. Only a few minerals have this level of fluorescence. Fluorite typically glows a blue-violet color under shortwave and longwave light.
How Many Minerals Fluoresce in UV Light?
Most minerals do not have a noticeable fluorescence. Only about 15% of minerals have a fluorescence that is visible to people, and some specimens of those minerals will not fluoresce. Fluorescence usually occurs when specific impurities known as "activators" are present within the mineral. These activators are typically cations of metals such as: tungsten, molybdenum, lead, boron, titanium, manganese, uranium, and chromium. Rare earth elements such as europium, terbium, dysprosium, and yttrium are also known to contribute to the fluorescence phenomenon. Fluorescence can also be caused by crystal structural defects or organic impurities.
In addition to "activator" impurities, some impurities have a dampening effect on fluorescence. If iron or copper are present as impurities, they can reduce or eliminate fluorescence. Furthermore, if the activator mineral is present in large amounts, that can reduce the fluorescence effect.
UV Lamps for Viewing
Scientific-grade lamps are produced in a variety of different wavelengths. They will often produce UV light in different ranges for UV-A (LW), UV-B (MW), or UV-C (SW) radiation. Most minerals react strongest under UV-C lights and always wear eye & skin protection to not burn yourself! (geology.com)
The Artist James Brunt
We have been waiting a few months for the right time to post this unique and awe-inspiring artist's work. Why did we wait you might ask? Well, because we thought his work was so unique and paid homage to the great stonemasons and neolithic builders of the past, as well as being something fun that any of us could do to emulate the wonder of what he generates. During this time that many of us are home, and perhaps are looking for ideas to do with our family or just for ourselves, we thought it would be a great time to post this.
If these inspire you, go out and create your own cairns and landscape-based art, and in turn, maybe you will inspire others too!




About the Artist
James Brunt creates elaborate ephemeral artworks using the natural materials he finds in forests, parks, and beaches near his home in Yorkshire, England. This form of land art, popularized and often associated with fellow Brit Andy Goldsworthy, involves detailed patterns, textures, and shapes formed using multiples of one kind of material. Brunt collects twigs, rocks, and leaves and arranges them in mandala-like spirals and concentric circles. He photographs his finished work to document it before nature once again takes hold of his materials. The artist frequently shares updates via Twitter and Facebook where he sometimes invites the public to join him as he works. Brunt also offers prints of his photographed artworks on his website.
Sources: http://www.jamesbruntartist.co.uk, https://www.thisiscolossal.com/2018/02/james-brunt-cairns-and-mandalas/
Scolecite
-Scolecite-195.jpg)


Figure A: Orangish Powellite, Apophyllite, and Scolecite
Figure B: Scolecite Spray (click to enlarge)
Figure C: Scolecite Crystal needles
Figure D: Scolecite and Stilbite Spray (click to enlarge)
Figure E: Scolecite Spray (click to enlarge)
Figure F: Pale Green Apophyllite with Scolecite from India
Figure G: Scolecite carved sphere. Sometimes Scolecite is carved into sculptures and more rarely carved into gemstones.




A
B
C
D
E
F
G
Here we have one of the extensive zeolite family of minerals that usually precipitates from warm mineralized fluids in volcanic settings, circulating in the Earth in heated convection cells. it was first described in 1813 and named after it’s pyroelectric reaction in the flame of a blowpipe (which used to be a standard mineralogical test way back when, in this case, a voltage is generated across the crystal when heated). It is a secondary mineral, growing in cavities within the lava such as gas bubbles. It also occurs in metamorphic rocks, when fluids released from higher temperature rocks below during their transformation by heat and pressure percolate upwards and precipitate their chemical contents upon encountering different temperatures or chemical conditions.
It usually grows as sprays of needle-shaped crystals (called acicular) with lines called striations running down the faces parallel to the long axis, or fibrous aggregates. The usual color is white but pink or salmony also occurs, as do red and green. Yellow or brown fluorescence is common, caused by electrons getting excited by UV light and jumping up an energy level, then releasing the energy as visible light. It can be very brittle despite its Mohs hardness of 5.5, so specimens should be handled and stored with great care to prevent damage and extraction from the hard mother rock is delicate at best.
The Technical Stuff
Scolecite is a tectosilicate mineral belonging to the zeolite group; it is a hydrated calcium silicate, CaAl2Si3O10·3H2O. Only minor amounts of sodium and traces of potassium substitute for calcium. There is an absence of barium, strontium, iron, and magnesium. Scolecite is isostructural (having the same structure) with the sodium-calcium zeolite mesolite and the sodium zeolite natrolite, but it does not form a continuous chemical series with either of them. It was described in 1813 and named from the Greek word, σκώληξ (sko-lecks) = "worm" because of its reaction to the blowpipe flame.
The structure of the aluminosilicate framework is the same for scolecite, natrolite, and mesolite. Scolecite has long ordered chains, rotated 24° around the axis of the chain. One Calcium cation and three water molecules are in four ion sites in the channels parallel to the C crystal axis. There is no sign of aluminum ions occupying silicon ion sites.
The Many Faces of Pyrite
Pyrite is a very interesting mineral. You might know it by its other name - Fool's Gold. It's heavy, often brassy golden in color and does all kinds of interesting things. Its crystals can be iridescent, perfect cubes, diamond-shaped, pentahedrons, pancakes, massive bulky geometric conglomerates, and can even form beautiful replacement casts of fossils, making them look like gold.


Radiating Pyrite in Rock Formation - Sun or Pancake
Pyrite Pentahedron with Quartz
Iron Pyrite is an iron sulfide with the chemical formula FeS2 (iron(II) disulfide). Pyrite is considered the most common form of sulfide minerals. Pyrite's metallic luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of fool's gold. The color has also led to the nicknames brass, brazzle, and Brazil, primarily used to refer to pyrite found in coal.
The name pyrite is derived from the Greek πυρίτης (pyritēs), "of fire" or "in fire", in turn from πύρ (pyr), "fire". In ancient Roman times, this name was applied to several types of stones that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what we now call pyrite. By Georgius Agricola's time, c. 1550, the term had become a generic term for all of the sulfide minerals.
Pyrite is usually found associated with other sulfides or oxides in quartz veins, sedimentary rock, and metamorphic rock, as well as in coal beds and as a replacement mineral in fossils, but has also been identified in the sclerites of scaly-foot gastropods. Despite being nicknamed fool's gold, pyrite is sometimes found in association with small quantities of gold.



Pyrite Fossil Replacement in Ammonites
Massive Geometric Pyrite Crystal
Pyrite Cube from Spain
Iron Pyrite is a fun crystal to collect. It is usually not very expensive and, as you can see from the above pictures, it comes in many fascinating forms and is a very beautiful metallic mineral. There's always room on the shelf for one more.
Strontium Titanate




What is Strontium Titanate?
Strontium Titanate is a man-made material. It grabbed public attention in the early 1950s as a diamond simulant - a material that has an appearance that is very much like diamond but has a different composition and/or crystal structure. It is one of those unique discoveries researched and developed by the National Lead Company. It had the cubic crystal structure and fire greater than that of a diamond. With this discovery, it looked like they had accomplished their goal. The result: SrTiO3 or Strontium Titanate and a material to simulate diamonds.
When cut and polished like a diamond, strontium titanate has a very similar luster, brilliance, and scintillation. However, strontium titanate has a "fire" that greatly exceeds the fire of a diamond. "Fire" is the ability of a gem to act as a prism and separate light passing through it into a rainbow of colors. The fire of strontium titanate is so strong that it immediately surprises the observer.
Between the early 1950s and the early 1970s, Fabulite, Diagem, and the other strontium titanate brands were popular sellers. Then, many people who purchased strontium titanate jewelry and wore it regularly began to notice that their stones were showing signs of wear. The facet faces were often scratched, and facet edges were often nicked and chipped. A material with a Mohs hardness of 5.5 does not stand up to wear like diamond with a hardness of 10, or ruby and sapphire with a hardness of 9.
Competition From Other Diamond Simulants
Strontium titanate does not have the hardness and toughness of diamond, and that was a problem. It only has a hardness of 5.5. That's low enough that contact with many common objects could result in a scratch or a damaged facet edge. This deficiency allowed newly developed simulants a place in the market.
Starting in the 1970s, simulants such as YAG (yttrium aluminium garnet), GGG (gadolinium gallium garnet) and cubic zirconia (CZ) quickly took market share away from strontium titanate. In the eye of many consumers, these simulants had an appearance that was similar to diamond and a durability that was superior to strontium titanate.
In the 1990s, synthetic moissanite began to replace YAG, GGG, and CZ in many of their uses. Its appearance is very similar to diamond, but it has a hardness and fire that is superior to all of these simulants from the 1970s. Cubic zirconia remains an important diamond simulant because its price is much lower than synthetic moissanite.
Today, strontium titanate is seldom seen in jewelry; however, it still has a more impressive fire than any natural or lab-created gemstones that are frequently seen in jewelry. It remains an attractive and satisfactory stone for earrings, pendants, and brooches that will encounter little abrasion or impact.
Tausonite - The Strontium Titanate Mineral
Naturally occurring strontium titanate was not known as a mineral until its discovery in 1982. It was first found in Eastern Siberia, Russia, and later occurrences were found in Paraguay and Japan. It is a very rare mineral, found in tiny cubic crystals, crystal clusters, and irregular masses. Natural specimens are typically so small and so rare that they have no commercial use beyond mineral specimens.
Comparison of Diamond Simulants
Material
-
Diamond
-
Strontium Titanate
-
YAG (Yttrium Garnet)
-
GGG (Gallium Garnet)
-
CZ (Cubic Zirconium)
-
Moisonite
-
White Sapphire
Refractive Index
2.147
2.41
1.83
1.97
2.2
2.66
1.77
Dispersion
0.044
0.190
0.028
0.045
0.060
0.104
0.018
Hardness
10
5.5
8.3
7
8.3
8.5 - 9.0
9.0
Strontium Titanate dispersion: The photos above show how strontium titanate has a spectacular dispersion when compared to moissanite, CZ, and diamond. Its dispersion is a little less than double that of moissanite, triple that of CZ, and more than quadruple that of diamond. In the photo above, the strontium titanate is a 6-millimeter round. The other stones are 4-millimeter rounds. This difference in size does give strontium titanate an advantage.
On a quick visual inspection, an experienced person will see that the dispersion of strontium titanate instantly stands apart from diamond, diamond simulants and moissanite. Strontium titanate sometimes contains bubbles that reveal its lab-created origin, and this distinguishes it from diamond. The much lower hardness of strontium titanate is usually obvious in jewelry that has been worn frequently as they exhibit levels of abrasion that are rarely seen in diamond, YAG, CZ, and moissanite.


The Giant Sea Scorpion
Giant Sea Scorpion Tracks Discovered in Scotland
330 million-year-old tracks made by a giant Arthropod, which was longer than a man, have been discovered in Fife (south-eastern Scotland). The trackway consists of three parallel lines representing the feet and in between a “scooped” out shape indicating that the tail was dragged; have been preserved in sandstone and were discovered by chance when Dr. Martin Whyte from the University of Sheffield was out walking.
The tracks have been ascribed to a sea scorpion called Hibbertopterus, fossils of which have been found in the area. Sea scorpions, or to be more precise Eurypterids (pronounced: You-ree-ip-ter-ids) were Chelicerate Arthropods that evolved around 480 million years ago and flourished worldwide in marine and freshwater environments until their demise towards the end of the Permian.
Fossils of Eurypterids are relatively common in ancient marine strata, as like Trilobites, they had to shed their body armor (exoskeleton) when they grew and the cast shells had a high preservation potential. Most Eurypterid fossils are not the fossilized carcass of a dead animal but instead are the fossilized remains of a cast-off shell from a molt.
Some types of Eurypterids grew to enormous sizes and until the rise of vertebrates such as fish, they were some of the top predators of the Palaeozoic. Click to read an article about the discovery of an enormous 3-meter long sea scorpion: Claws! Giant Sea Scorpion of the Devonian
This Scottish discovery is the largest known walking trackway of an Arthropod, or indeed any invertebrate discovered to date.


Size Comparison and Artist Rendering of the Giant Sea Scorpion
Image Credit: Bristol University
Image Credit: Wikipedia Commons
The tracks were probably made as this huge animal hauled itself out of the water. Eurypterids, with their simple gills, were adapted to absorb oxygen from both water and the atmosphere. It is likely these animals moved into the shallow waters in order to breed, just like a relative of these creatures, the Horseshoe Crab does today.
The tracks are already quite badly eroded but removing the sandstone rock in which they are preserved may be too difficult. Instead, Scottish Natural Heritage is funding a project to create silicone copies of the trackway which will enable these ancient “footprints” to be studied in detail. A spokesperson for Scottish Natural Heritage, described this discovery as “unique and internationally important because the creature was gigantic.”
Richard Batchelor from Geoheritage Fife, commented:
“The trackway is in a precarious situation, having been exposed for years to weathering. The rock in which it occurs is in danger of falling off altogether. Removing it and housing it in a museum would be prohibitively costly but molding it in silicone rubber and making copies for educational and research purposes means that we can still see and research this huge creature’s tracks in years to come.”
The sandstone has been dated to approximately 330 million years ago (mid-Carboniferous). This area of eastern Scotland is world-famous for its Carboniferous fossil sites. For example, at East Kirton, a number of important fossil-rich Mississippian (Lower Carboniferous) strata are known. It seems that around 330 million years ago, this area of Scotland was low lying with many freshwater lakes. Many early Tetrapod fossils, as well as numerous invertebrate fossils and plants, are known from this region.
Sources: https://blog.everythingdinosaur.co.uk/blog/_archives/2010/04/21/4512505.html, https://www.thoughtco.com/tetrapods-facts-129452, https://fr.m.wikipedia.org/wiki/Fichier:Jaekelopterus_rhenaniae_reconstruction.jpg, https://blog.everythingdinosaur.co.uk/blog/_archives/2007/11/24/3373385.html
Gemmy Fossils Reveal New Dinosaur

Image Credit: James Kuether
Four members of this newly described plant-eater were found together in what may be Australia’s first known dinosaur herd.
Unusually colorful fossils found in Australia belong to a stunning new species of plant-eating dinosaur, scientists report today in the Journal of Vertebrate Paleontology. The remains not only belong to the first herd of dinosaurs discovered in the country, but they also represent the most complete dinosaur fossil yet found preserved in opal.
Discovered near the town of Lightning Ridge, about 450 miles northwest of Sydney, the hundred-odd bones have a rare blue-grey hue with occasional flashes of brilliant gem-quality color. Lightning Ridge is famous for yielding fossils hewn from often brightly colored opal, a gemstone that forms over long periods from the concentration of silica-rich solutions underground. But finding a whole new dinosaur species is remarkable.
“Any time we find a new Australian dinosaur it’s interesting because we have so few,” says Stephen Poropat, a paleontologist from Swinburne University of Technology in Melbourne who was not on the study team. The tally of known Australian dinosaurs is currently around 24, he notes, including Weewarrasaurus, another species from Lightning Ridge described last year.
The newest species, Fostoria dhimbangunmal, was an Iguanodon-like dinosaur that lived about a hundred million years ago during the mid-Cretaceous period when this region was a broad floodplain with lakes and rivers flowing into the inland Eromanga Sea. “The floodplains were frequently wet and richly vegetated, meaning they were a good place for plant-eating dinosaurs,” says study leader Phil Bell, a paleontologist at the University of New England in Armidale, New South Wales.
Studying dinosaurs from the time slice at Lightning Ridge is important, Poropat adds, as the world was then experiencing the warmest conditions of the past 150 million years. “These dinosaurs were living in a really incredible greenhouse Earth,” he says. “The globe would have potentially looked quite different, and these fossils can tell us how these dinosaurs were coping.”

Right: This fossil is part of a vertebra from the back of a Fostoria dinosaur.
Left: This fossil toe bone belonged to a member of Fostoria dhimbangunmal. The fossils were all found in a former opal mine and show glimmers of the brilliantly colored gemstone.

Bundle of Bones
Long-time Lightning Ridge opal miner Bob Foster found the fossil in 1986. Scientists at Sydney’s Australian Museum, along with Australian Army reservists, helped Foster excavate the find as an accumulation of dinosaur bones embedded in blocks of rock, with the museum then taking the fossils into their collections.
But the fact they were left languishing unstudied for 15 years or so and put on display at a Sydney opal store led Foster to decide to reclaim his discovery. He returned it to Lightning Ridge, and his family eventually donated it to a local museum, the Australian Opal Centre, where Bell was able to study the find.
“We originally thought it was one skeleton, but once we began to study the individual bones, we realized … there were parts of four scapulae, or shoulder blades, all of different sizes,” he explains. About 60 of the bones are from a probable adult that was 16 feet in length, while the others are from juveniles of various sizes, prompting Bell to speculate that they were the remains of either a family or small herd of herbivorous dinosaurs.
“We have bones from all parts of the body, but not a complete skeleton,” he says. “These include bones from the ribs, arms, skull, back, tail, hips, and legs. So, it’s one of the most completely known dinosaurs in Australia … [with] 15 to 20 percent of the skeleton of the species.”
The name Fostoria honors Bob Foster, while the species name dhimbangunmal means ‘sheep yard’ in the local Yuwaalaraay and Yuwaalayaay Aboriginal languages. It was chosen by Foster’s wife Jenny, who is Gamilaraay Aboriginal, to honor the Sheepyard locality where Foster’s, now defunct mine once operated.

Image Credit by [cisiopurple]
Developing Duckbills
About the length of an elephant, Fostoria would have habitually walked on its hind limbs, though the scientists surmise it sometimes used all four to get around. It likely ate primitive plants called horsetails as well as bunya and hoop pines, fossils of which are also found in the region. (Find out about a sauropod dinosaur that likely crawled as a baby and walked on two legs as an adult.)
A relative of Iguanodon and Australia’s most famous dinosaur, Muttaburrasaurus, Fostoria is also an early member of a group that would elsewhere evolve into the duckbilled hadrosaurs, which were common in North America and Asia toward the end of the time of the dinosaurs, roughly 66 million years ago.
“Early duckbill dinosaurs were the primordial soup from which the fantastical crested species … later evolved,” says Lindsay Zanno, a paleontologist at the North Carolina Museum of Natural Science in Raleigh who was not involved in the research.
“Although the pace of discovery of early duckbills like Fostoria has intensified around the globe, we still have much to learn about how these herbivores became so successful,” she adds.
Image Credit: Evgenia Arbugaeva/National Geographic Magazine

The New Ivory Trade
We held off a bit before publishing this article because we had a speaker come in to discuss the extinction of Woolly Mammoths and Mastodons. We wanted to see what additional information he would shed on this subject before we wrote on the subject.
While it is true that the buying and selling of Ivory tusks from Elephants has been outlawed and is illegal in most countries with international trade being largely shut down, black market ivory selling still goes on and much of it goes to China. However, there is a new source that is being poorly tracked and is a tricky subject. This is the new market for selling Mastodon and Mammoth Ivory on the Black Market. Some see this as much more ethical that poaching live elephants, and we would agree with that sentiment, but its also robbing the scientific community the chance to learn why these animals became extinct.
Basically, in isolated regions of Russia's Siberia, Ivory hunters are traveling by sea, land, and air to get to the frigid areas that have been melting away rapidly over the last two decades. Many of these hunters are very poor, have little opportunity for work, and have hungry mouths to feed at home.
The most likely cause for the recent melting of the permafrost is global climate change brought about by man's activities. In reality, as the permafrost thaws, it releases more and more greenhouse gasses into the atmosphere creating more melting and hotter temperatures around the world.
Siberian Tusk Hunters use water hoses and horses to cut away at the frozen ground to dig deep holes into the soils causing it to melt much faster to excavate caves and hunt for tusks and Woolly Rhinoceros horn. Rhinoceros horn is valued like gold in Eastern Countries because folk medicine believes that it has powerful medicinal qualities. A five-pound Rhino horn can sell for over $11,000 to wholesalers in Vietnam.
The hunters risk their lives in mosquitos infested forests, frigid temperatures, remote locations and crawl back into caves, that are starting to collapse as soon as they dig them out, for a chance at a small fortune in the new ivory trade.

The Mammoth tusk this man is carrying will be worth about $34,000. Image Credit: Amos Chapple

Photo Credit: Associated Press. A Complete Mammoth skeleton sold for over $645,000 in 2017.
Ivory Artifacts

Woolly Mammoth Pistol Grip from Ivory found in Alaska.


Mammoth molar sold as is. These teeth are sometimes slabbed to make knife handles, jewelry and other objects of art.

Ivory artifacts from the Yanaste Complex in Siberia. Shown here are spear points, needles, a scoop, bracelet and diadem. Our ancestors had an affinity for ivory as well.
Carmeltazite - Harder than Diamonds?
Extraterrestrial Mineral Harder than Diamonds Discovered in Israel
Original Article by Helen Flatley in the Vintage News

A new discovery in the mountains of northern Israel has caused significant excitement for geologists around the world. While working in the Zevulun Valley, close to Mount Carmel, Israeli mining company Shefa Yamim found a new mineral never before discovered on earth.
The International Mineralogical Association regularly approves new minerals for its official list, with up to 100 new substances added to the register each year. However, this latest discovery was hailed as a significant event, as it was previously believed that this type of mineral was only found on extraterrestrial material. The new mineral substance has been found to naturally occur on Earth.
The CEO of Shefa Yamim, Abraham Taub, told Haaretz that the mineral had been named carmeltazite, after the place of its discovery. The elements contained within its structure: titanium, aluminum, and zirconium were previously found in allendeite, a mineral seen on the Allende meteorite that fell to earth in February of 1969.
While the majority of the new minerals approved by the International Mineralogical Association are unspectacular in appearance, carmeltazite offers considerable commercial opportunities, as it resembles other gemstones used in the making of jewelry.
This strange new mineral was found embedded in cracks within sapphire, the second hardest mineral (after diamonds) found to occur naturally on earth. Carmeltazite closely resembles corundum (sapphire or ruby) in its chemical composition and is found in black, blue-green, or orange-brown colors, with a metallic hue. However, after density testing, scientists discovered that carmeltazite is even denser than diamond, and is significantly scarcer, making its value extremely high.
According to the BBC, the region close to the Savulun Valley in Isreal is known for volcanic activity dating from the Cretaceous period. The Carmel range is home to at least 14 volcanic vents that created the geological conditions for the formation of carmeltazite, over extremely long periods. It is thought that carmeltazite formed 18 miles under the surface of the earth, close to the crust-mantle boundary. High pressure and temperatures produce partially molten rocks that release fluids and react to form new minerals. As vents emerge in the surface of the earth, this volcanic matter is rapidly transported into the upper crust along with other materials, creating the type of deposits found in Mount Carmel.
The mining company has been working intensively in this region due to the possibilities offered by this rich geological legacy. Although they were principally looking for sapphire, the new mineral was discovered embedded in the gemstones they harvested and had analyzed. The mining company has recovered many samples but carmeltazite remains extremely rare. The largest stone discovered to date reached 33.3 carats. Haaretz reports that the mineral has been trademarked by the mining company as “Carmel Sapphire” and it has recently been approved as a new mineral by the International Mineralogical Association’s Commission on New Minerals.
Although the Commission regularly approves new discoveries, it is unusual to find a substance so spectacular in appearance and quality, and a result has attracted a significant amount of international attention. To date, carmeltazite has only been discovered in the Zevulun Valley, which means it is one of the rarest minerals in the world and is also likely to be one of the most expensive.
CEO Taub stated that the company intends to market the mineral as a gemstone, and potentially use it in the production of high-end jewelry. One thing is sure: this mineral is likely to command a monumental price tag when it eventually hits the market.
But is it harder than Diamonds?
The original reports that MMS could locate do not specify the hardness of the new mineral. It is, in fact, a variant of corundum (an Aluminum Oxide) with elements of Titanium and Zirconium. Both Titanium and Zirconium Oxides are hard, and the mineral Carmeltazite is denser than diamonds, but it's unlikely to be harder than diamonds. On Mindat.org the chemical formula is listed as ZrAl2Ti4O11. This combination of chemical bonds will more likely place Carmeltazite closer to 8.7 to 9.4 on the mols scale with the Titanium-Zirconium elements. However, there is a big difference between 9 and 10 in the mols hardness scale. Zirconium-Titanium alloys are known of in metallic forms, but being that carmeltazite is formed in heavy pressures and heat, perhaps it will have other properties in wear resistance or tensile strength that will lead to new discoveries?

Proposed Chemical Structure of Carmltazite
Rainbow Flame Obsidian
Beautiful to behold and even more amazing when polished into cabochons and items, Rainbow Flame Obsidian is subtle and complex in its color sheen. There are few words to describe its unique characteristics other than it's got a really cool name to go along with its labradorescent like color play.
Rainbow Obsidian is obsidian volcanic glass that has multicolored iridescence caused by inclusions of magnetite nanoparticles. The magnetite particles interfere with light that passes into the semi-transparent mineral structure and creates the rainbow sheen. While Obsidian is often thought of as nature's glass or black volcanic glass, there are a number of varieties that collectors are interested in finding.
Some varieties include Mahogany Obsidian, Snowflake Obsidian, Golden Sheen Obsidian and the topic this month of Rainbow Obsidian. Consequently, Rainbow Obsidian comes in several varieties based on their color quality, similar to Opals. Some Rainbow Obsidian names are Velvet Peacock, Flame, Banded, and Iris Obsidian, to name a few. It has been cut and polished into jewelry pieces for centuries and is famously sharp when knapped. Modern-day flint knappers have discovered this amazing varietal and are producing some stunning artifacts as we will show you below.
Rainbow Obsidian is coming mostly from Mexico and Oregon that is available in the United States. Rainbow Obsidian comes from three very specific places. In Lake County, Oregon from the Glass Buttes, the Modoc Plateau near Davis Creek, California, and La Revoltosa Mine in Jalisco Mexico. Generally speaking, Obsidian is not an expensive collector's item but the vibrant colors in Flame Obsidian will fetch a premium price. It also becomes more expensive outside of North America and is best used for trading with other collectors from other countries who don't see it as much as we do here.
Obsidian is a natural glass and may have razor-sharp edges that can easily cut skin and flesh. Handle with care. Do not grind this mineral dry since long-term exposure to finely ground powder may lead to silicosis.

Rough Rainbow Iris Obsidian Rock

Velvet Flame Obsidian Arrowhead

Knapped Rainbow Obsidian Spearhead


Oregon Fire Obsidian


Velvet Peacock Obsidian
Archaeologists in Spain Discover a Cache of
Prehistoric Crystal Artifacts
Archaeologists in Spain have unearthed an extremely rare set of weapons, including a long dagger blade, twenty-five arrowheads, and cores used for creating the artifacts, all made of crystal! The finding was made inside megalithic tombs dating to the 3rd millennium BC in the southwest of Spain.
An excavation of megalithic tombs in Valencina de la Concepción in Spain led to the dramatic discovery of the rare relics, which experts described as exceptional and magnificently well-preserved. The objects are estimated to be over five thousand years old (dating back to at least 3000 BC). As the Daily Grail writes, the Montelirio tholos, excavated between 2007 and 2010, is a great megalithic construction which extends nearly 44 meters (144 ft) in total, constructed out of large slabs of slate. At least 25 individuals were found within the structure. Analyses suggested that there was one male and numerous females who had drunk a poison substance. The remains of the women sit in a circle in a chamber adjacent to the bones believed to be of their chief.
There are no crystal mines in the nearby proximity which suggests that the creators of these objects must have traveled many hundreds of kilometers to source their material. The scarcity of crystal rock in addition to the enormous amount of craftsman the construction of the artifacts involved suggests that these were elite products. Given that the crystal weapons were in a tomb, it suggests that they were used as highly sought after funerary items given to select individuals for ceremonial purposes.


Both Images: The crystal dagger blade. © Morgado, A., et al.
The blade is 214 mm in length, a maximum of 59 mm in width and 13 mm thick.
In addition to the human remains and textiles, ivory tusks and carvings, the archaeologists found the large hoard of crystal arrowheads. The fact that they were discovered altogether indicates that they could have been a ritual offering at an altar. The arrowheads have the distinctive long lateral appendices of flint arrowheads from the region, but archaeologists noted that even greater skill must have been required to produce these unique features when using rock crystal.
The predictable conchoidal (semi-circular) fracture patterns you get from flint, chert, and obsidian make them fantastic materials to work. Crystals of this sort have a vastly different microscopic crystalline structure and would behave completely differently when struck. I can’t imagine where you would even start to develop the proper technique except for many years of trial and error.

A: Ontiveros arrowheads;
B: Montelirio tholos arrowheads;
C: Montelirio dagger blade;
D: Montelirio tholos core;
E: Montelirio knapping debris;
F: Montelirio micro-blades;
G: Montelirio tholos micro-blades;
Photograph: Miguel Angel Blanco de la Rubia.
The Sad and the Amazing
The area that was excavated held secrets to an undiscovered civilization that lived in the area that had built a large settlement at least 5000 years ago. These tools and other artifacts are currently being studied and are considered priceless because they point to an unknown culture that existed in Western Spain leading to additional speculation into an advanced, unknown people who inhabited, not only the Iberian peninsula, but branched out into African and the Atlantic Ocean long before historians have documented its existence. Sadly, the small teams of Archaeologists had to file injunctions to stop construction over the site of shopping malls, apartments, and parking lots. As a result, they could only study the site for three years.
Interestingly though, despite being found relatively frequently in burials of the 4th and 3rd millennia BCE, crystal implements disappear from later funerary monuments in the Early Bronze Age (beginning of the 2nd millennium BCE) - a "truly striking" development, researchers say, as it would seem "the use of this raw material as grave goods was almost entirely abandoned", although the reasons remain a mystery.
How to tell the Difference between Jasper, Agate, Chalcedony, and Geodes
If you read about the gem materials used for lapidary work and rock tumbling, you will encounter three names over and over again. These are "Agate", "Jasper", and "Chalcedony." These names are often misunderstood and often used incorrectly. With a little knowledge, you can use these names correctly for most specimens. However, some specimens can be difficult or impossible to name correctly with these terms if you must rely only on visual inspection of the material.
We would like to provide a short lesson on these names to help you understand them and use them correctly - as much as that is possible. The simple answer is if you put a light behind the material and you can see through it, then it is an Agate and if you can’t, then your holding Jasper. The more complex answer is that it is not always that straightforward. The simple science behind this question is that both Agates and Jaspers are comprised of Quartz - which is one of the most common minerals on the planet. Quartz is comprised of two major types: macrocrystalline (large crystal) and cryptocrystalline or microcrystalline (small crystal).

What is Chalcedony?
Chalcedony is a generic name given to materials that are composed of microcrystalline quartz. Agate and jasper are both varieties of chalcedony.
What is microcrystalline quartz? "Quartz" is a mineral composed of silicon and oxygen (SiO2) and the word microcrystalline means that the quartz is in the form of crystals that are so small that a microscope must be used to visualize them individually. Sometimes the word "cryptocrystalline" is used instead of "microcrystalline" but both of these words are imprecise ways of communicating a microscopic size.
Chalcedony is a very hard material. It has a hardness of 7 on the Mohs scale. It breaks with a conchoidal fracture, and freshly broken pieces have a very smooth, non-granular texture and a waxy to vitreous luster. These characteristics enable chalcedony to be cut and polished into bright, durable gemstones. Varieties of chalcedony are favorite materials for making tumbled stones.
Chalcedony occurs in a wide range of colors. It is commonly gray, white, brown, red, yellow, orange and black, but it can occur in any color. It can also be banded or have plume, dendritic, mottled, mossy or other colorful structures contained within its structure. At one time the word "chalcedony" was reserved in parts of the gemstone industry for a light blue translucent material; however, this use of the word has nearly disappeared.
Chalcedony includes Carnelian, Chrysoprase, Agate, Bloodstone, Jasper, and others. When Chalcedony is in concentrically banded patterns it is called an Agate. Occasionally the banding is larger than the crystal and the banding is not visible- like with most Carnelian.
Light Blue Chalcedony Tumbled Stones

What is Agate?
Agate is translucent to a semi-transparent form of chalcedony. If you have a piece that is semi-transparent you will be able to hold a very thin piece up and see distorted or foggy images through it. If you hold a translucent piece up to a source of light you will see a small amount of light passing through the thin edges.
Agate is generally a banded material, and observing bands in a specimen of chalcedony is a very good clue that you have an agate. However, some agates do not have obvious bands. These are often translucent agates with plume-shaped, dendritic or mossy inclusions.
Many agates form in areas of volcanic activity where waters, rich in dissolved silica (SiO2), flow through fractures and cavities in igneous rocks. When the solution is highly concentrated with dissolved silica, a silica gel can form on the walls of these cavities. That gel will slowly crystallize to form microcrystalline quartz.
Over time, additional layers of gel are deposited and these form younger bands of microcrystalline quartz on the walls of the cavity. If the dissolved mineral composition of the silica-rich water changes over time, impurities (elements other than silicon and oxygen) can be incorporated into the gel and into the microcrystalline quartz. These impurities can alter the color of the microcrystalline quartz. This can produce the color banding. Crystallization of foreign materials is often what forms the plumes, dendrites, or mossy structures that are often seen in translucent agate.
Although agates typically form in igneous rocks such as basalt, rhyolite, and andesite, they can also form in sedimentary rocks such as limestone. All of these types of rock are more susceptible to weathering than agate. So when the rocks are eventually broken down by weathering, the durable agates will remain. This is why agate nodules are often found in stream valleys that cut through fine-grained igneous rocks or limestone.
Lake Superior Agates

What is Jasper?
Jasper is an opaque variety of chalcedony. Opaque means that light does not pass through.
Microcrystalline quartz in its pure form is semi-transparent. When a small amount of impurities or foreign materials are added, the color of the microcrystalline quartz changes and its ability to transmit light decreases. Jasper contains enough impurities and foreign material to render it opaque. So, the real difference between Jasper and agate is the number of impurities and foreign material contained with a specimen.
Jasper is an opaque rock of virtually any color stemming from the mineral content of the original sediments or ash. Patterns arise during the consolidation process forming flow and depositional patterns in the original silica-rich sediment or volcanic ash. Hydrothermal circulation is generally thought to be required in the formation of Jasper.
Jasper can be banded or striated, depending on how it formed, and are most commonly red, yellow, green, brown or a mixture of these colors. The banding in agate is based on periodic changes in the translucency of the agate substance. Layers appear darker when they are more translucent (this may appear reversed in transmitted light). This effect may be accompanied and amplified by changes in the color of neighboring layers, due to other co-precipitated minerals.
While agate is typically a material that forms in the cavities of igneous rock or limestone, Jasper often forms when fine particulate materials are cemented by silica. This often occurs in soft sediments when silica precipitates and cements them into a solid mass. These included particles are what give Jasper its color and opacity. A sedimentary material is known as chert forms in extensive bedded deposits. It is also an opaque variety of chalcedony that can be called a "Jasper."
Jaspers are also known to form when volcanic ash or fine pyroclastics are cemented into a solid material from the precipitation of silica from solution. The cementation process is sometimes so aggressive that the sediment, ash or volcanic particles are dissolved or recrystallized into microcrystalline quartz.
Jaspers of Several Colors

What is a Geode
Geodes are rocks that are hollow inside, rather than solid all the way through. Geodes are generally round, though some are egg-shaped. They can range from the size of a nut to several feet. Most geodes are approximately the size of a basketball. When broken or cut open, geodes reveal a lining of crystals or other materials inside. Many of these crystals can be quite beautiful, such as the purple quartz known as amethyst. Some geodes even contain liquid petroleum. Calcite geodes contain white crystals, but sometimes these can be other colors, and under fluorescent light, additional colors show up. Other examples of geode interiors include celestite, agate, smoky quartz, and rose quartz. Chalcedony is a common mineral coating for many geodes, and it is permeable to water over time. Anhydrite geodes have interiors that resemble cauliflower. Other examples of minerals found in geodes include gypsum, calcite, dolomite, pyrite, ankerite, celestite, aragonite and goethite.
The System of Assigning a Name
Chalcedony is a name that is based upon two things: 1) crystal size, and 2) composition. Chalcedony is microcrystalline quartz. Easy!
Agate is a name based upon three things: 1) crystal size, 2) composition and 3) how light passes through the mineral. Agate is microcrystalline quartz with a translucent to semitransparent diaphaneity. Easy!
Jasper is a name based upon three things: 1) crystal size, 2) composition and 3) diaphaneity. Jasper is microcrystalline quartz with an opaque diaphaneity. It is opaque because it contains enough non-chalcedony material to interfere with the passage of light. Easy. Jaspers can be confusing to identify as well from the multiple colors and names that they are sold under and some names are not consistent from place to place.
Geode is a rock that is hollow on the inside. The only way to find out for sure if a rock is a geode is to break it apart by tapping it with a hammer, or have someone cut open the rock with a powerful saw. You'll know once you see the interior and whether or not there is a hollow or solid composition. The hollow ones are geodes, and as mentioned before, are often lined with crystals or layers of minerals. Some geodes are highly sought after and can be polished after being cut.
If you have a piece of chalcedony, determining if it is an agate or a jasper is easy when that material is clearly semitransparent, translucent or opaque. However, it can be difficult to determine the boundary between translucent and opaque. In addition, some specimens can have translucent zones and opaque zones. What are they called? Some people have solved this problem by using the term "jaspagate" or "jasper-agate" when a specimen contains zones ofboth jasper and agate. Anyone who is correctly using the name "jaspagate" has probably given a rock more than a casual look.
Mucking things up a bit...
Dalmatian Stone: The material is known as "dalmatian stone" because it is a white rock with lots of black spots, has often been called "dalmatian jasper." However, we sent some out for analysis and learned that it was not Jasper at all, but an igneous rock composed of tiny grains of white feldspar and black grains of a hornblende group mineral. Dalmatian stone is often dyed, so if you see a stone with black spots and an outrageous color (like the blue, red, green and purple stones at right) it might be dalmatian stone.
Picasso Stone: This material is known as "Picasso stone" because it looks like an abstract painting. Many people also call it "Picasso Jasper". However, this material is not a "Jasper". It is actually dolomite (a dolomitic marble to be more precise) that is mined in Utah. Dolomite is a carbonate rock that is very different from quartz. It is very soft for a gem material with a hardness of only 4 on the Mohs scale.
Ocean Jasper: The material known as "ocean jasper" is reported to be silicified rhyolite - another igneous rock. Ocean Jasper is a really interesting material mined in the country of Madagascar (an island nation off the southeastern coast of Africa. If you look at it closely, many pieces will contain concentric orbs, translucent banded agate, opaque jasper, and vugs lined with druzy quartz crystals. It is a "Jasper" and much more!
Petrified Wood: We don't want to start any arguments, but much of the material called "petrified wood" is composed of chalcedony with an opaque diaphaneity. Shouldn't that make it "jasperized wood" or at least a variety of Jasper? Something to ponder. Many people don't realize that petrified wood is found at many locations from around the world. Petrified wood for rock tumbling can be a mix of samples from Arizona to China or a mix of petrified wood from many locations in the United States.
Green Tree Agate and Green Moss Agate: These materials are often genetically related - that means they form under similar conditions. Green moss agate is a semitransparent agate with green mossy inclusions inside. Green tree agate is a white jasper with green mossy inclusions inside - however, they are only visible where they are exposed at the surface. Green moss agate and green tree agate have been found together in the same deposit - having formed just a short distance from one another.
Bumblebee: This exceptionally colored material is often called "bumblebee jasper" or "eclipse jasper". It forms on the island of Bali near the hot vents of an active volcano named Mount Papandayan. As a banded material, and for that reason, some people want to call it "agate." It is an opaque material, and for that reason, some people want to call it Jasper. However, it is neither. It is a lithified sediment that contains a volcanic brew of materials that include: volcanic ash, gypsum, barite, sulfur, and even some orpiment (an arsenic mineral!). It is often cut into cabochons, but much of what is cut is stabilized with resin because it is soft, porous and fragile. Not recommended for rock tumbling!






Veszelyite
Veszelyite is a rare, but beautiful copper and zinc phosphate mineral. Specimens often command some fairly lofty prices for even diminutive specimens, which is a direct testament to veszelyite's rarity and attractiveness. It has a nice emerald-green to green-blue color and a high luster which produces a good colorful sparkle. Crystals are often randomly and individually attached on specimens much like green sprinkles that are spread on an ice cream cone. larger Crystal of Veszelyite can look very much like Azurite or other copper-bearing minerals. It has the distinctive blue-green color so many other copper ores. It also can resemble Dioptase and Viviandite in form and color.
Veszelyite falls in the phosphate group of minerals with a chemistry of (Cu, Zn)3PO4(OH)3 - 2H2O, Hydrated Copper Zinc Phosphate Hydroxide. It is really only valued for its rarity as a mineral specimen and its usually associated with quartz, zinc secondary compounds and copper ores like malachite and hemimorphite.
It was named after the Hungarian engineer A. Veszelyi (1820-1888) who discovered the species. It is a fairly soft mineral with a Mohs hardness of about 3.5 to 4, so it is not suitable for jewelry. It is found in Kipushi, Shaba, Rep. of Congo; Kabwe, Zambia; Moravicza, Banat, Romania, and the Black Pine Mine, Montana, USA.







Image Credit: Brad Zylman

The Little Fossil that CoulD
This Mesosaurus fossil was obtained legally from Brazil back in the 1960s and remained in a personal collection for several decades until it was donated to MMS in 2017. Because the fossil was broken across its vertical center, MMS had the fossil stabilized (you can see the crack in the picture). There are only a few of these fossils in circulation and to find one is a very good day.
Our little Mesosaurus lived during the early Permian Period in what is now Africa and South America. If you look at Mesosaurus pictures, you may jump to the conclusion that this animal was some sort of prehistoric crocodile. After all, it does kind of look like one. However, that would be the wrong conclusion to make. That’s because, while this reptile looked very much like a crocodile and lived a semi-aquatic lifestyle, its teeth give its true identity away. This animal’s teeth were very thin and were used to filter plankton and not to bite into fish or small animals. Not to mention the fact that it was quite a bit smaller than most of the prehistoric crocodiles that would come later.
An adult Mesosaurus was approximately 3 feet long and weighed around 20 pounds. That made it the size of a yardstick and about the weight of a Dachshund dog. It was an anapsid reptile – which means it didn’t have the openings in the sides of its skull that therapsids and pelycosaurs had. It was in the same classification category as turtles are today. It would roam the shoreline of rivers and lakes looking for small marine organisms and would occasionally get in the water to eat its favorite food: plankton. It would catch the plankton by filtering fresh water through those oddly shaped teeth.
One of the most fascinating facts about Mesosaurus, however, is the fact that it was instrumental in proving continental drift theory. It lived in both eastern South America and southern Africa. However, since it only swam in fresh water, it is highly unlikely it could have crossed the Atlantic Ocean to get from South America to Africa. This most likely means that these two areas were connected at one point in time and over time, spread apart. The theory that is known as continental drift changed how we see geology and our world today.


Story Credits: Wikipedia.com, newdinosaurs.com, Brad Zylman




Musgravite
A Rare and Wondrous Gemstone
Originally discovered in 1967, the dark and stormy stone known as Musgravite comes in on our list of rare gemstones you’ve probably never heard of. It certainly isn’t as well-known as rubies and sapphires, but you can expect to pay a steep price for this gorgeous example of Mother Nature’s talents.
The high pricing can be attributed to the stone’s rarity. Musgravite was first found in the late sixties and was named for the area of Australia in which it was discovered – the Musgrave Ranges.
The gorgeous color of this gemstone is formed when there are the perfect percentages of magnesium, iron, and zinc present. Because it requires such precise conditions, the stone is far from common. That rarity, combined with an undeniable beauty makes this a very pricey gem. You can expect to pay approximately $6,000 or per carat. In some instances, the price has been pushed to as much as $35,000 per carat. For many, it is considered a worthwhile investment, because the stone is so gorgeous – ranging from a deep gray to a soft purple. Some variations could be mistaken for amethyst, a much more common and less-expensive gemstone. However, do not be fooled. Musgravite is its own entity and worthy of the praise it has received through the years. Furthermore, it is much harder than amethyst, which is a member of the quartz family. While quartz is generally graded at a 6.5 to 7, Musgravite is ranked at an 8 or 8.5, which places it closer to topaz on the scale. That also means that this is a very durable stone that would be well placed in a ring or bracelet setting, able to hold up to regular wear and tear.
Musgravite is a rare mineral closely related by composition to the mineral taaffeite. This magnesium-rich beryllium oxide crystallizes in the trigonal system, in contrast to the hexagonal system of taaffeite, and is highly sought after by rare stone collectors. A 0.86 ct musgravite, identified by Raman spectroscopy, contained a particularly interesting inclusion scene consisting of numerous etch tubes (see above center image) that broke the surface of the faceted stone. With a direct source of light, these etch tubes displayed vibrant colors resulting from thin-film iridescence in the air-filled, crystallographically aligned tubes. This is the first musgravite gemstone displaying any type of optical phenomenon that anyone has examined to date.
Musgravite Statistics:
Musgravite or magnesiotaaffeite - (chemical formula of Be(Mg, Fe, Zn)2Al6O12), is a rare oxide mineral. Its locality is the Ernabella Mission, Musgrave Ranges, South Australia for which it was named. It is a member of the taaffeite family of minerals. Its hardness is 8 to 8.5 on the Mohs scale. The closely-related Magnesiotaaffeite, which crystallizes in the hexagonal system, is known in mineralogy as Magnesiotaaffeite-2N’2S. Together, they are both parts of the Taaffeite group.
The rare gems taaffeite and musgravite have lately become more popular among collectors. Due to their similar chemical compositions and crystal structures, their main gemological properties overlap and so sophisticated measurement techniques such as quantitative chemical analysis, Raman spectroscopy, X-ray powder or single crystal diffraction are needed for their identification. A special rotating and tilting stage has been constructed to non-destructively determine the differences in diffraction pattern based on the different symmetries (trigonal and hexagonal), unit cell dimensions and space groups of taaffeite and musgravite.
Facet grade musgravite was not reported until 1993 and as of 2005, there were only eight musgravite specimens, three of which were identified by Murray Burford, a Canadian gemologist. The mineral has since turned up in Greenland, Madagascar, Antarctica, Sri Lanka, and Tanzania.
Credits: Wikipedia.com, https://www.diamondrocks.co.uk, www.gia.com, http://www.musgravite.com, http://bjordangemstones.blogspot.com, mindat.org

Sound waves reveal diamond cache deep in Earth’s interior
Study finds that 1–2% of Earth’s oldest mantle rocks are made from diamond ...
Jennifer Chu | MIT News Office
July 16, 2018
There may be more than a quadrillion tons of diamond hidden in the Earth’s interior, according to a new study from MIT and other universities. But the new results are unlikely to set off a diamond rush. The scientists estimate the precious minerals are buried more than 100 miles below the surface, far deeper than any drilling expedition has ever reached.
The ultradeep cache may be scattered within cratonic roots — the oldest and most immovable sections of rock that lie beneath the center of most continental tectonic plates. Shaped like inverted mountains, cratons can stretch as deep as 200 miles through the Earth’s crust and into its mantle; geologists refer to their deepest sections as “roots.”
In the new study, scientists estimate that cratonic roots may contain 1 to 2 percent diamond. Considering the total volume of cratonic roots in the Earth, the team figures that about a quadrillion tons of diamond are scattered within these ancient rocks, 90 to 150 miles below the surface.
“This shows that diamond is not perhaps this exotic mineral, but on the [geological] scale of things, it’s relatively common,” says Ulrich Faul, a research scientist in MIT’s Department of Earth, Atmospheric, and Planetary Sciences. “We can’t get at them, but still, there is much more diamond there than we have ever thought before.”
Faul’s co-authors include scientists from the University of California at Santa Barbara, the Institut de Physique du Globe de Paris, the University of California at Berkeley, Ecole Polytechnique, the Carnegie Institution of Washington, Harvard University, the University of Science and Technology of China, the University of Bayreuth, the University of Melbourne, and University College London.
A Sound Glitch!
Faul and his colleagues came to their conclusion after puzzling over an anomaly in seismic data. For the past few decades, agencies such as the United States Geological Survey have kept global records of seismic activity — essentially, sound waves traveling through the Earth that are triggered by earthquakes, tsunamis, explosions, and other ground-shaking sources. Seismic receivers around the world pick up sound waves from such sources, at various speeds and intensities, which seismologists can use to determine where, for example, an earthquake originated.
Scientists can also use this seismic data to construct an image of what the Earth’s interior might look like. Sound waves move at various speeds through the Earth, depending on the temperature, density, and composition of the rocks through which they travel. Scientists have used this relationship between seismic velocity and rock composition to estimate the types of rocks that make up the Earth’s crust and parts of the upper mantle, also known as the lithosphere.
However, in using seismic data to map the Earth’s interior, scientists have been unable to explain a curious anomaly: Sound waves tend to speed up significantly when passing through the roots of ancient cratons. Cratons are known to be colder and less dense than the surrounding mantle, which would in turn yield slightly faster sound waves, but not quite as fast as what has been measured.
“The velocities that are measured are faster than what we think we can reproduce with reasonable assumptions about what is there,” Faul says. “Then we have to say, ‘There is a problem.’ That’s how this project started.”
Diamonds in the Deep
The team aimed to identify the composition of cratonic roots that might explain the spikes in seismic speeds. To do this, seismologists on the team first used seismic data from the USGS and other sources to generate a three-dimensional model of the velocities of seismic waves traveling through the Earth’s major cratons.
Next, Faul and others, who in the past have measured sound speeds through many different types of minerals in the laboratory, used this knowledge to assemble virtual rocks, made from various combinations of minerals. Then the team calculated how fast sound waves would travel through each virtual rock, and found only one type of rock that produced the same velocities as what the seismologists measured: one that contains 1 to 2 percent diamond, in addition to peridotite (the predominant rock type of the Earth’s upper mantle) and minor amounts of eclogite (representing subducted oceanic crust). This scenario represents at least 1,000 times more diamond than people had previously expected.
“Diamond in many ways is special,” Faul says. “One of its special properties is, the sound velocity in diamond is more than twice as fast as in the dominant mineral in upper mantle rocks, olivine.”
The researchers found that a rock composition of 1 to 2 percent diamond would be just enough to produce the higher sound velocities that the seismologists measured. This small fraction of diamond would also not change the overall density of a craton, which is naturally less dense than the surrounding mantle.
“They are like pieces of wood, floating on water,” Faul says. “Cratons are a tiny bit less dense than their surroundings, so they don’t get subducted back into the Earth but stay floating on the surface. This is how they preserve the oldest rocks. So we found that you just need 1 to 2 percent diamond for cratons to be stable and not sink.”
In a way, Faul says cratonic roots made partly of diamond makes sense. Diamonds are forged in the high-pressure, high-temperature environment of the deep Earth and only make it close to the surface through volcanic eruptions that occur every few tens of millions of years. These eruptions carve out geologic “pipes” made of a type of rock called kimberlite (named after the town of Kimberley, South Africa, where the first diamonds in this type of rock were found). Diamond, along with magma from deep in the Earth, can spew out through kimberlite pipes, onto the surface of the Earth.
For the most part, kimberlite pipes have been found at the edges of cratonic roots, such as in certain parts of Canada, Siberia, Australia, and South Africa. It would make sense, then, that cratonic roots should contain some diamond in their makeup.
“It’s circumstantial evidence, but we’ve pieced it all together,” Faul says. “We went through all the different possibilities, from every angle, and this is the only one that’s left as a reasonable explanation.”
This research was supported, in part, by the National Science Foundation.

Olivine grains that eroded from lava on Papakolea Beach, Hawaii - Photography by Professor Mark A. Wilson
Need another reason to travel to Hawaii? How about tiny gemstones falling out of the sky? This is just another reason why Hawaii is a magical and unique place unlike any other on Earth. So for this month we are going to focus on olivine, a mineral that makes up vast amounts of the Earth's subsurface. It comes in a number of varieties, but it is commonly known as Peridot when used as a gemstone.
Hawaii's Kilauea volcano has been fiercely erupting for well over a month and residents are finding little green gems that have fallen out of the sky during Kilauea's eruption. The green gems are olivine crystals, a common mineral found in Hawaii's lava. At jewelry quality, the mineral is called peridot. As the volcano erupts, it blasts apart molten lava, allowing for green olivine minerals to be separated from the rest of the melt and fall as tiny gemstones.
There are several places in Hawaii that the beaches are a green color due to the high concentrations of olivine that has weathered out of the mafic lava (basalt). In fact, olivine is one of the most common minerals below Earth's surface but it is quite hard to find it separated from the parent rock and even harder to find it of gem quality.
Olivine is so common that experts estimate over 50 percent of Earth's upper mantle is composed of olivine or variations of the mineral. While it is a common mineral, little crystals of olivine falling out of the sky are quite unusual.
"Friends of mine live in Hawaii, right next to the area impacted by the most recent lava flows. In the midst of the destruction nearby & stress of the unknown, they woke up to this - tiny pieces of olivine all over the ground. It is literally
raining gems. Nature is truly amazing." — Erin Jordan (@ErinJordan_WX) June 11, 2018
Hawaii's volcanoes are hotspots, where the mantle magma continually upwells and burns a hole through Earth's oceanic crust. Oceanic crust, in composition, is very similar to what we would find in the upper mantle with high olivine concentrations. This means the true composition of the upper mantle is not significantly altered when erupted on Hawaii's surface as basalt. The reason we get a variety of other rocks on continents is largely due to magma traveling through the varied geology that underlies each continent. This adds and removes chemicals/minerals and alters the original composition of the magma from basalt to a unique blend of minerals.
Olivine can be found throughout the island, typically as a mineral within the basalt rock. However, from the continuous pounding of waves or construction, the minerals can be broken away from the surrounding basalt. Typically, olivine erupts with the calm oozing of basalt lava on Hawaii, locking it away within the rock fabric. However, in this instance the sudden ejection of lava into the air rapidly cools and separates the melt, allowing the olivine to lithify as a separate crystal.
While it may seem like a perfect excuse to buy a ticket to Hawaii and collect some gems, please note that removing rocks, minerals, or sand from Hawaii is not only in poor taste, it is illegal. So please make sure to enjoy them in the moment, take photos, but leave the beauty on the island for others to enjoy in the future.
Only time will tell how the uniquely prolonged eruption plays out and what next will dazzle us from Hawaii's Kilauea volcano.
Kilauea Volcano Eruption
Papakolea Green Sand Beach





Trillion Cut Peridot
Peridot Rough Crystal
Kilauea's Little Olivine Gems

A colorized image, enlarged 100,000 times, shows an ultra-thin layer of molybdenum disulfide stretched over the peaks and valleys of part of an electronic device.
Nature loves crystals. Salt, snowflakes and quartz are three examples of crystals – materials characterized by the lattice-like arrangement of their atoms and molecules.
Industry loves crystals, too. Electronics are based on a special family of crystals known as semiconductors, most famously silicon. To make semiconductors useful, engineers must tweak their crystalline lattice in subtle ways to start and stop the flow of electrons. Semiconductor engineers must know precisely how much energy it takes to move electrons in a crystal lattice.
This energy measure is the band gap. Semiconductor materials such as silicon, gallium arsenide and germanium each have a band gap unique to their crystalline lattice. This energy measure helps determine which material is best for which electronic task.
Now an interdisciplinary team at Stanford has made a semiconductor crystal with a variable band gap. Among other potential uses, this variable semiconductor could lead to solar cells that absorb more energy from the sun by being sensitive to a broader spectrum of light. The material they used is in itself not new. They chose molybdenum disulfide, or MoS2, a rocky crystal like quartz, that is refined for use as a catalyst, a coating for turning things black and as a lubricant.
Molybdenum disulfide is what scientists call a monolayer: A molybdenum atom links to two sulfurs in a triangular lattice that repeats sideways like a sheet of paper. The rock found in nature consists of many such monolayers stacked like a ream of paper. Each MoS2 monolayer has semiconductor potential.
“From a mechanical engineering standpoint, monolayer MoS2 is fascinating because its lattice can be greatly stretched without breaking,” said Zheng, an associate professor. By stretching it to different tolerances, different electrical properties can be engineering into the monolayers.
Based on a 2012 MIT theoretical paper, the team at Stanford created a silicon landscape that they could sculpt in exquisite detail and then bathed the nanoscale hills and valleys in a monolayer of molybdenum disulfide.
By stretching the lattice, the Stanford researchers were able to shift the atoms in the monolayer. Those shifts changed the energy required to move electrons around. Stretching the monolayer made MoS2 something new to science and potentially useful in electronics: an artificial crystal with a variable electronic conduction.
Molybdenum disulfide is an inorganic compound composed of molybdenum and sulfur. Its chemical formula is MoS2. The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as the mineral molybdenite, the principal ore for molybdenum. MoS2 is relatively unreactive. In appearance and feel, molybdenum disulfide is similar to graphite. It is widely used as a dry lubricant because of its low friction and robustness. Bulk MoS2 is a diamagnetic, indirect bandgap semiconductor similar to silicon.

Molybdenum Disulfide Mineral Sample
Molybdenum Disulfide Chemical Model - 2 Monolayers
Image Credits: Electron micrograph photo - Hong Li, https://en.wikipedia.org/wiki/Molybdenum_disulfide, http://pubs.rsc.org/-/content/articlelanding/2017/cp/c6cp07302f/unauth#!divAbstract

Covellite
Sources: Wikipedia, Mindat.org, https://www.gemsociety.org
Covellite (also known as covelline) is a rare copper sulfide mineral with the formula CuS. This indigo blue mineral is ubiquitous in copper ores, it is found in limited abundance and is not an important ore of copper itself, although it is well known to mineral collectors. The mineral is associated with chalcocite in zones of secondary enrichment (supergene) of copper sulfide deposits. Commonly found with and as coatings on chalcocite, chalcopyrite, bornite, enargite, pyrite, and other sulfides, it often occurs as pseudomorphic replacements after other minerals. Despite the very rare occurrence as a volcanic sublimate, the initial description was at Mount Vesuvius by Nicola Covelli (1790–1829).
Covellite is quite beautiful as a natural specimen exhibiting Indigo-blue or darker, inclining towards blue-black, often iridescent with purplish, deep red, and brassy-yellow reflections in its color spectrum. In addition to its own beauty, it is often found with pyrite and other sulfides that can make stunning specimens.
Covellite is commonly found as a secondary copper mineral in deposits. It is rarely a primary mineral in copper deposits, and is even less likely to be found as a volcanic sublimate. Covellite is known to form in weathering environments near the surface in deposits where copper is found with sulfides. As a primary mineral, the formation of covellite is restricted to hydrothermal
conditions.
Covellite's occurrence is widespread in the United States. In Silver Bow County, Montana, covellite has been found in veins at depths of 1,150 m (3,770 ft), as the primary mineral. Covellite formed as clusters in these veins reaching one meter across in Leonard mines, Montana. As a secondary mineral, covellite also forms as descending surface water in enrichment zone oxides and redeposits covellite on some sulfides (pyrite and chalcopyrite). Locally, findings of covellite have been discovered in salt domes and at the McCellan copper mine in Foard County, Texas. An unusual occurrence of covellite was found replacing organic debris in the red beds of New Mexico.
It has also been reported from the Calabona mine, Alghero, Sardinia; at Bor, Serbia; from Leogang, Salzburg, Austria; at Dillenburg, Hesse and Sangerhausen, Saxony, Germany; from Kedabek, Caucasus Mountains, Russia and in the Bou-Skour mine, Bou Azzer district, Morocco.
Covellite was the first identified naturally occurring superconductor. The framework of CuS3/CuS2 allow for an electron excess that facilitate superconduction during particular states, with exceptionally low thermal loss. Material science is now aware of several of covellite's favorable properties and several researchers are intent on synthesizing covellite. It has been experimentally demonstrated that the presence of ammonium vanadates is important in the solid state transformation of other copper sulfides to covellite crystals.
As a gemstone or in jewelry applications, covellite is a poor choice because of its low hardness (1.5-2.0 Mols) but it is sometimes made into cabochons or more rarely it is faceted because of it's beautiful peacock blue colors. Covellite is often found with pyrite and chalcopyrite which can add to its beauty in cabs. These polished stones are often protected with a jewelers sealant that is much harder than the covellite to protect it from easily being scratched or broken.









Covellite Cabochon
Covellite Cabochon
For February we are going to take a short trip down memory lane to discover Michigan Meteorites. With the recent hit that Michigan took, we thought it would be very timely to explore the known history of Meteorite impacts in the Great Lakes State. For those of you who are unfamiliar with what a meteor is, it's a piece of an asteroid, comet or other planet that has been been captured by Earth's gravity and falls through the sky to be found.
Michigan is No Stranger to Meteorites
Credit to Mark Torregrossa | mtorregr@mlive.com
Michigan has had at least 10 meteorites found on its soil. Historical records show where the meteorites were found and how much they weighed. Meteorites are named after the location they are found. It the case like last week, the meteorite will likely be named after where the biggest piece was found. The most recent meteorite hasn't been named yet.
Meteorites are also considered a "find" or a "fall." A fall is a meteorite that is found right after falling, and the location can be pegged to a certain falling meteor. The most recent meteorite will be classified a fall because we saw it one night and found pieces in the following days. Other meteorites are classified as finds. The typical found meteorite was picked up in a farm field as ground was being cleared in Michigan.

Michigan's Known Meteorites
In this graphic are the locations of meteorites found or seen falling in Michigan. Information is derived from 'Meteorites of Michigan', a MSU Abrams Planetarium publication, and John Zawiskie, curator of Earth and Life Sciences at Cranbrook Institute of Science, Bloomfield Hills, MI.
Image Credit: mlive
Grand Rapids - 112 Lb. Meteorite
According to "Meteorites of Michigan" by MSU's Abrams Planetarium, the Grand Rapids meteorite was the biggest and the first significant meteorite found in Michigan. It was found in 1883, three feet underground. The meteorite weighed 112.4 pounds. It was composed of 89 percent iron, 10 percent nickel and some traces of other elements.
Allegan - 70 Lb. Meteorite
A 70 pound meteorite fell near the village of Allegan on July 10, 1889. It fell at 8 a.m. and was wintessed by Michiganders. One account says a child and his siblings were weeding a potato field when they heard it fall. At the time, people thought it was 20 feet in the ground, but it was only 1.5 feet in the ground. They were told to wait until the next day to dig it out because it would be too hot.
Reed City - 44 Lb. Meteorite
The Reed City meteorite weighed almost 44 pounds and was found in a farm field. Pieces of the meteorite are displayed at Michigan State University, Chicago Natural History Museum and several other museums.
Seneca - 4.2 Lb. Meteorite
In 1923 a meteorite was found in a corn field in Seneca township. This meteorite was traced back to 1903 using personal journals written on the farm. The meteorite was tested for composition and was mostly iron and nickel with trace amounts of cobalt, copper, sulphur and phosphorus.
Worden Meteorite - Only Damaging Sky Stone
In 1997 a meteorite actually hit a garage and car in Worden, MI. The meteorite was small in size, but blasted right through the roof of a garage. A model of this meteorite and damage to the car are on display at Cranbrook Institute of Science. The meteorite in Worden was traveling so fast it made a clean hole in the garage roof. The hole mirrored the shape of the meteorite. John Zawiskie, of the Cranbrook Institute of Science, compared the hole to cartoons when a character goes through a wall and leaves an exact body print.
Iron River - 3.1 Lb. Meteorite
According to "Meteorites of Michigan," the composition brought scientists to date the meteorite at 290 million years old to 430 million years old. They determined the meteorite originated from a cosmic collision over 300 million years ago.
Coleman - 1.1 Lb. Meteorite
The Coleman meteorite was only 1.1 pounds and was found in 1994. This meteorite is classified a "fall" because the meteorite was found just after a fireball hit the ground.
A Michigan meteorite caught on camera in the Michigan thumb area.


Images of the new Michigan Meteorite
The Recent Michigan Chondrite Meteorite
credit: Mark Hicks, The Detroit News
On Tuesday, January 16th at 8:15p.m., a six foot wide meteor broke apart over the earth at about 20 miles high. The Majority of the fragments seem to have landed in Hamburg Township. Numerous people either saw the meteor fall, saw the flash of light, felt the tremors or heard a loud explosion like sound that evening. “The asteroid hit the atmosphere, moving about 28,000 miles per hour, broke apart over southeast Michigan and scattered meteorites on the ground,’’ said Bill Cooke, lead of the NASA meteoroid environment office.
The first fragments were located Thursday by professional meteorite hunters Larry Atkins and Robert Ward of Arizona, according to the American Meteor Society. Ward used seismic data, radar information and eye witnesses to track down the pieces he was able to find. Those remnants must undergo analysis at a laboratory accredited to certify meteorites, such as Chicago’s Field Museum. Hunters had started combing southeast Michigan for the pieces the day after a NASA camera spotted the meteor over Ohio.
The new meteor has already been classified as a chondrite: A chondrite has a stony composition that contains small granules of minerals (usually silicate minerals). Chondrites are physically and chemically the most primitive meteorites in the solar system. Because the meteor burned up during it's path through the atmosphere, there is a black fusion crust on some of the samples found so far. It takes a ton of patience to find any fragments and the recent snow melt will make it more difficult to find more pieces as the first pieces found were located on top to the snow which helped them to stand out.
Meanwhile, Darryl Pitt, a curator at Macovich Collection of Meteorites in New York and meteorite consultant to the Christie’s auction house, is offering $20,000 to the first person willing to sell a recovered chunk weighing at least 2.2 pounds (1 kilogram). “I want to motivate more people to look,” Pitt, a Michigan native, said Thursday night. “If you take every meteorite known to exist, take the total weight, they still weigh less than the world’s annual output of gold. Meteorites are extraordinarily rare and the world is just coming to terms with how special they are.”
Those heading out to net the nuggets should beware!
Federal law says meteorites belong to the owners of the property where they land. Testing must determine whether the find qualifies as one, and even if that’s the case, “no meteorite is worth millions of dollars,” said Bill Cooke, who leads NASA’s meteoroid environment office in Alabama. “Meteorites, like gold, are prized according to type and weight.”
Figures can vary depending on circumstances, including the market and collectors’ interest, but prices for the first found and certified pieces could start at $100 per gram if more examples are not found and it remains rare. Even so, scientists seek the rocks to learn more about the phenomenon. Every meteorite is a special prize of science and the solar system. The monetary value is secondary.” That’s why Atkins and his team plan to keep searching for more artifacts. “These rocks are virgin materials from the building blocks of our solar system,” he said. “When you’re holding a meteorite, you’re holding something. It’s not just a rock.”
All meteorite hunters need to ask permission before entering any private property.
This months featured mineral is one of those that has a mysterious name. It is one of the first geodes that sparked many people to start collecting minerals and learning how crystals form.
Septarian
The name Septarian is derived from the Latin name, Septem, meaning seven. This relates to the fact that the mud balls cracked with 7 points in every direction, thereby creating the beautiful design.
Septarians were formed during the Cretaceous period, 50 to 70 million years ago when the Gulf of Mexico reached what is now Southern Utah. Decomposing sea life killed by volcanic eruptions, had a chemical attraction for the sediment around them, forming mud balls. As the ocean receded, the balls were left to dry and crack. Because of their bentonite content they also shrank at the same time trapping the cracks inside. As decomposed calcite from the shells was carried down into the cracks in the mud balls, calcite crystals formed. A thin wall of calcite was transformed into aragonite separating the bentonite heavy clay exteriors from the calcite centers. Because of this, the nodules are called Septarians.
Septarians are composed of Calcite (The Yellow Centers), Aragonite (The Brown Lines) and the Outer Grey Rock is basically Limestone. Occasionally the fossil or some of the fossils which started the formation of the rock is noticeable in the rock.
Septarian is a special type of concretion. Concretions are masses of mineral matter formed when minerals in water are deposited about a nucleus (such as a leaf or shell or other particle) forming a rounded mass whose composition or cement is usually different from the surrounding rock. This can occur at the time of deposition, shortly thereafter, or after the sediment has hardened.
Generally, concretions are harder than the rocks around them; therefore, over time the concretions can weather out of the surrounding rocks. Concretions in Kansas are formed from any of a number of minerals, including calcite, limonite, barite, pyrite, or silica. They vary widely in shape and size, with the huge spherical concretions at Rock City in Ottawa County and Mushroom Rock State Park in Ellsworth County measuring up to 27 feet in diameter.
When the concretion is exposed to weathering, the softer parts between the calcite-filled cracks are eroded and the cracks extend above the surface of the concretion, like ridges or little walls.
These concretions are actually pseudo-fossils (think "fossilized" ripple marks, raindrop hits, etc...) in this case what you have are "fossilized" mud cracks. These formed when the lake bed dried out. As the mud of the lake bottom dried it contracted and voids formed. The lake bed was subsequently covered with new lake and the older one was buried. The water from the new lake seeped down through the sedimentary rock of the old lake and picked up trace amounts of calcium in passing. when the water entered the voids it would pool and the calcium would deposit out as aragonite and calcite. Most collectors label their Septarian specimens as "Septarian nodule", since the piece is composed of multiple minerals.
These nodules are often large and have interesting calcite crystal chambers. The colorful golden yellows and aragonite deep browns will contrast with the fossilized clay matrix creating artistic designs. As a result, Septarians are carved into spheres, hearts, bookends, animals and sometimes complex sculptures. See some of the the examples below:

Septarian Slab

Septarian Slab with Exposed Calcite Chamber

Septarian Carved Face

Egg with Calcite Pocket

Septarian Skull Sculpture











